7 EDTA, Tetraso
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EDTA, Tetrasodium Tetrahydrate Salt
EDTA, Tetrasodium Tetrahydrate Salt sc-204735 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME EDTA, Tetrasodium Tetrahydrate Salt STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Chelating/sequestering agent for the clarification of liquids, pharmaceuticals, detergents, shampoos, agent for metal poisoning, decontamination of radioactive liquids and in analytical chemistry. Used in agricultural chemical sprays; metal cleaning and plating; to remove insoluble deposits of magnesium and calcium salts; in textiles to improve dying; scouring and detergent operations; decreasing blood cholesterol. SYNONYMS C10-H12-N2-Na4-O8, (OOCCH2)2NCH2CH2CH2N(CH2COO).Na4, "Tetracemate tetrasodium", "Versene 220", Aquamollin, "Edathanil tetrasodium", Tetracemin, Sequestrene, Complexon, Tyclarosol, "Endrate tetrasodium", Tetrine, Irrgalon, "Nervanaid B", Questex, Kalex, Calsol, Warkeelate, "edate terasodium", "Trilon B", Nervanoid, Aquamoline, "tetrasodium EDTA", Komplexon, "Syntes 12a", "tetrasodium edetate", Nullapon, Chemcolox, Conigon, ethylenebis(imminodiacetate), -

EDTA (Ethylenediaminetetraacetic Acid) C Barton, Oak Ridge Institute for Science and Education, Oakridge, TN, USA
EDTA (Ethylenediaminetetraacetic Acid) C Barton, Oak Ridge Institute for Science and Education, Oakridge, TN, USA Ó 2014 Elsevier Inc. All rights reserved. This article is a revision of the previous edition article by C. Charles Barton and Harihara M. Mehendale, volume 2, pp 147–148, Ó 2005, Elsevier Inc. l Name: EDTA (Ethylenediaminetetraacetic Acid) depending on the salt. Algae and invertebrates are among the l Chemical Abstracts Service Registry Number: 60-00-4 most sensitive species based on predictive modeling for acute l Synonyms: Ethylenedinitrilotetraacetic acid; Celon A; and chronic endpoints for EDTA, depending on the compound. Cheelox; Edetic acid; Nullapon B Acid; Trilon BW; Versene EDTA and its salts also do not appear to be very toxic for l Molecular Formula: C10H16N2O8 terrestrial wild mammals, and adverse effects from reasonably l Chemical Structure: expected agricultural uses are not expected. According to ChemIDPlus, EDTA has the following physi- OH cochemical properties: melting point ¼ 245 C, pKa dissocia- tion constant ¼ 0.26, log P (octanol–water) ¼3.860, water À solubility ¼ 1000 mg l 1, vapor pressure ¼ 4.98E-13 mm Hg, O O À Henry’s law constant ¼ 1.17E-23 atm-m3 mol 1, and atmo- ¼ 3 À1 N OH spheric OH rate constant 1.82E-10 cm molecule-s . HO N Based on its physicochemical properties, EDTA is not expected to volatilize from soil or water. When released to the O O atmosphere, EDTA should sorb to particulate matter, and appears to have the potential to photolyze. OH Exposure and Exposure Monitoring Background Exposure to EDTA may be through FDA-approved uses as food Ethylenediaminetetraacetic acid (EDTA) was developed by Franz additives, in sanitizing solutions, in pharmaceutical products, Munz in Germany during the 1930s as an alternative to citric acid. -

Lessons and Considerations for the Creation of Universal Primers Targeting Non-Conserved, Horizontally
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.30.017442; this version posted April 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Lessons and considerations for the creation of universal primers targeting non-conserved, horizontally 2 mobile genes 3 4 Damon C. Brown,a Raymond J. Turnera# 5 aDepartment of Biological Sciences, University of Calgary, Calgary, Alberta, Canada 6 7 Running Head: Primer design approaches for nonconserved mobile genes 8 #Address correspondence to Raymond J. Turner, [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.30.017442; this version posted April 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 9 Abstract 10 Effective and accurate primer design is an increasingly important skill as the use of PCR-based 11 diagnostics in clinical and environmental settings is on the rise. While universal primer sets have been 12 successfully designed for highly conserved core genes such as 16S rRNA and characteristic genes such as 13 dsrAB and dnaJ, primer sets for mobile, accessory genes such as multidrug resistance efflux pumps 14 (MDREP) have not been explored. Here, we describe an approach to create universal primer sets for 15 select MDREP genes chosen from five superfamilies (SMR, MFS, MATE, ABC and RND) identified in a 16 model community of six members (Acetobacterium woodii, Bacillus subtilis, Desulfovibrio vulgaris, 17 Geoalkalibacter subterraneus, Pseudomonas putida and Thauera aromatica). -

Report Worst Ingredients in Liquid Soap Italian Products
Date Dec 9, 2011 Report Worst Ingredients in Liquid Soap Italian products EuroConsumers EnviroPlanning AB Lilla Bommen 5 C, SE-411 04 Göteborg, Sweden Visitor address Lilla Bommen 5 C Telephone +46 31 771 87 40 Telefax +46 31 771 87 41 Web site www.enviroplanning.se e-mail [email protected] Worst Ingredients in Liquid Soap Date Dec 9, 2011 Italian products Version Final Doc.no 4084-01\10\01\Report.doc About the report Title Worst Ingredients in Liquid Soap Italian products Version 001 Date December 9, 2011 Client Euroconsumers Servizi Editoriali S.R.L. Via Valassina, 22 20159 MILANO Italy Project number 4084-01 Document number 4084-01\10\01\Report.doc Cover photo Helena Norin Report written by Helena Norin and Niklas Hanson Report reviewed by Jenny Robinson Report verified by Helena Norin U:\4084-01\10-udo\01-utr\Report 4084-01.doc I (III) Worst Ingredients in Liquid Soap Date Dec 9, 2011 Italian products Version Final Doc.no 4084-01\10\01\Report.doc Summary Euroconsumers is preparing a review of liquid soaps on the Italian market. One part of this review looks at the environmental properties of the ingredients in the chosen products. EnviroPlanning has been hired to assess the environmental properties of the ingredients in order to identify and list ten or more of the most un-wanted ingredients. Severe health aspects of the ingredients are also taken into account when establishing the list. The following ingredients are considered as the most unwanted in the examined 27 soaps: BHT (butylated hydroxytoluene) Methylchloroisothiazolinone Zinc Oxide Methylisothiazolinone Limonene Lauric Acid 2-Bromo-2-Nitropropane-1,3-Diol Benzyl Benzoate Butylphenyl Methylpropional Disodium EDTA Formaldehyde The review shows that the liquid soaps on the Italian market have a wide range of ingredients. -

List of Ingredients
List of PROHIBITED Ingredients Ace-K (acesulfame potassium) Glycerol Ester of Wood Rosin Aluminum Calcium Silicate/Bentonite High Fructose Corn Syrup (HFCS) (calcium aluminosilicate, calcium L-cysteine silicoaluminate, sodium calcium silicoaluminate) Methyl Silicon Aluminum Potassium Sulfate/ Microparticulated Whey Protein Aluminum Sulfate Concentrate Ammonium Chloride MSG (monosdodium glutamate)* Artificial Colors Nitirites/ Nitrates Artificial Flavors Neotame Aspartame Parabens (all) Astaxanthin* Polydextrose Azodicarbonamide Potassium Bromate AZO Dyes Potassium Lactate Benzoates Potassium Sorbate Benzoic Acid Propionates (calcium and sodium) Benzoyl Peroxide (synthetic only) Propyl Gallate (PG) Benzyl Alcohol (synthetic only) Saccharin/Calcium Saccharin BHA (butylated hydroxyanisole) Salatrim BHT (butylated hydroxytoluene) Sodium Aluminum Phosphate Bromated Flour Sodium Aluminum Sulfate Brominated Vegetable Oils/ BVO Sodium Diacetate (except in beverages) Sodium Erythorbate Calcium Bromate Sodium Glutamate Calcium Peroxide Sodium Lactate Calcium Sorbate Sodium Lauryl Sulfate (SLS) Calcium stearoyl-2-lactylate Sodium Stearoyl-2-Lactylate Canthaxanthin* Sorbic Acid Caprocaprylobehenin Stannous Chloride Carboxymethyl Cellulose Sucralose Carmine Sucroglycerides Certified Colors/ FD&C Colors Sucrose Polyester (olestra) Cochineal Sulfur Dioxide Cyclamates TBHQ (tertiary butylhydroquinone) DATEM Tetrasodium EDTA Dimethylpolysiloxane Theobromine* Dioctyl Sodium Sulfosuccinate (DSS) Titanium Dioxide Disodium Guanylate (GMP) Triacetin/Glycerol Triacetate Disodium Inosinate (IMP) Vanillin EDTA/Calcium Disodium EDTA Sulfites** Ethyoxyquin Excludes select beverages, cookies and Rice Crispies. As we continually strive to evolve and improve, our list of prohibited ingredients is subject to change. *Except where occurring naturally **Allowable in wines and vinegars. -

Concord Food Co-Op Unacceptable Ingredients for Food
Concord Food Co-op Unacceptable Ingredients for Food (as of April 15, 2021) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

Short Review of Calcium Disodium Ethylene Diamine Tetra Acetic Acid As a Food Additive
European Journal of Nutrition & Food Safety 4(4): 408-423, 2014 SCIENCEDOMAIN international www.sciencedomain.org Short Review of Calcium Disodium Ethylene Diamine Tetra Acetic Acid as a Food Additive Marijke M. H. Van De Sande1, Sabrina Wirtz1, Ellen Vos1 and Hans Verhagen2,3* 1Maastricht University, Department of Human Biology, PO Box 616, 6200 MD Maastricht, The Netherlands. 2National Institute for Public Health and the Environment (RIVM), PO Box 1, 3720 BA Bilthoven, The Netherlands. 3University of Ulster, Northern Ireland Centre for Food and Health (NICHE), Cromore Road, Coleraine, BT52 1SA Northern Ireland. Authors’ contributions This work was carried out in collaboration between all authors. Authors HV and SW designed the work. Author MHVDS conducted the literature research, analyzed the data and wrote the first version. Authors HV, SW and EV were responsible for subsequent reviewing and scientific editing, while author MHVDS was the primary responsible for final content. All authors read and approved the final manuscript. The authors declare no conflicts of interest and this research received no grant from any funding agency. Received 26th March 2014 th Mini Review Article Accepted 24 June 2014 Published 14th July 2014 ABSTRACT Calcium disodium ethylenediaminetetraacetate (Calcium Disodium EDTA, C10H12CaN2Na2O8.2H2O) is a derivative of EthylenediamineTetraacetic Acid and is an approved food additive (E385). It is used as preservative, sequestrant, flavouring agent, and colour retention agent in foods. As a drug it is used for the reduction of blood and mobile depot lead in the treatment of acute and chronic lead poisoning. Calcium Disodium EDTA is very poorly absorbed from the gastrointestinal tract following ingestion. -

Antiseptics on Bacteria in Subcutaneous Chlorhexidine
J Clin Pathol: first published as 10.1136/jcp.12.1.48 on 1 January 1959. Downloaded from J. clin. Path. (1959), 12, 48. A TECHNIQUE FOR STUDYING THE ACTION OF ANTISEPTICS ON BACTERIA IN SUBCUTANEOUS TISSUES, WITH SPECIAL REFERENCE TO CHLORHEXIDINE BY A. R. MARTIN From the Research Department, Imperial Chemical Industries Limited Pharmaceuticals Division, Alderley Park, Macclesfield, Cheshire (RECEIVED FOR PUBLICATION JULY 3, 1958) Bacteria which gain access to a freshly inflicted Zinnemann (1947) removed an ellipse of skin, wound are at first external to the tissues and do contaminated the raw area with streptococci, and not immediately cause any reaction. During this treated it by dropping on antiseptic solutions. stage, which is commonly said to last up to about These methods have failed to control one or more six hours, the wound is described as contaminated, of the following variables: (1) The number of and the organisms in it may be susceptible to organisms implanted in or on the tissues; (2) the antiseptics. Later, when bacteria have invaded amount of antiseptic in contact with the the tissues, the wound is described as infected, and contaminated tissues; (3) the length of time for locally applied antiseptics are at a great dis- which the antiseptic acts. The third point is copyright. advantage. The great differences which exist (and particularly difficult to meet, because any form of the difficulties in defining them) between accidental bandaging of small animals is almost impracticable. wounds in man and animals in respect of the age It is believed that the method described below, and nutritional status of the subject on the one whilst not imitating the treatment of traumatic hand, and the nature, site, severity, and degree of wounds, goes far towards controlling the above contamination of the wound on the other, have variables. -

Full Circle Market List of 104 Ingredients
Full Circle Market List of 104 Ingredients 1. ACESULFAME-K (ACESULFAME POTASSIUM) 36. DISODIUM CALCIUM EDTA 71. POTASSIUM BISULFATE 2. ACETYLATED ESTER OF MONO-AND 37. DISODIUM DIHYDROGEN EDTA 72. POTASSIUM BROMATE DIGLYCERIDES 3. AMMONIUM CHLORIDE 38. DISODIUM GUANYLATE 73. POTASSIUM HYDROXIDE 4. ANTIBIOTICS 39. DISODIUM SUCCINATE 74. POTASSIUM METABISULFITE 5. ARTIFICIAL FLAVORS 40. EDTA (ETHYLENEDIAMINE TETRAACETIC 75. POTASSIUM NITRATE OR NITRITE ACID) 6. ASPARTAME 41. ERYTHORBIC ACID 76. POTASSIUM SORBATE (ACCEPTABLE IN SUPPLEMENTS ONLY) 7. ASTAXANTHIN (ACCEPTABLE IN 42. ESTER GUMS 77. PROPIONATES (CALCIUM AND SODIUM) SUPPLEMENTS, IF NATURAL) 8. AZODICARBONAMIDE 43. ETHYL VANILLIN 78. PROPYL GALLATE 9. BENTONITE (ACCEPTABLE IN 44. ETHYLENE OXIDE 79. PROPYLENE OXIDE SUPPLEMENTS) 10. BENZOATES IN FOOD 45. ETHOXYQUIN 80. PROPYLPARABEN 11. BENZOYL PEROXIDE 46. FD&C BLUE NO. 1 81. SACCHARIN 12. BENZYL ALCOHOL 47. FD&C BLUE NO. 2 82. SIMPLESSE 13. BHA (BUTYLATED HYDROXYANISOLE) 48. FD&C GREEN NO. 3 83. SODIUM ALUMINUM PHOSPHATE 14. BHT (BUTYLATED HYDROXYTOLUENE) 49. FD&C RED NO. 3 84. SODIUM ALUMINUN SULFATE 15. BISULFITES 50. FD&C RED NO. 40 85. SODIUM BENZOATE 16. BLEACHED FLOUR 51. FD&C YELLOW NO. 5 86. SODIUM BISULFATE 17. BROMATED FLOUR 52. FD&C YELLOW NO. 6 87. SODIUM DIACETATE 18. BROMINATED VEGETABLE OIL (BVO) 53. GLYCEROL ESTER OF WOOD ROSIN 88. SODIUM FERROCYANIDE 19. CALCIUM BROMATE 54. HEXA-, HEPTA- AND OCTA-ESTERS OF 89. SODIUM GLUTAMATE SUCROSE 20. CALCIUM DISODIUM EDTA 55. HIGH FRUCTOSE CORN SYRUP 90. SODIUM METABISULFITE 21. CALCIUM PEROXIDE 56. HYDROGENATED OIL 91. SODIUM NITRATE/NITRITE 22. CALCIUM PROPIONATE 57. HYDROXYPROPYL GUAR GUM 92. -

RECENT DEVELOPMENTS in WOUND ANTISEPTICS by H
Postgrad Med J: first published as 10.1136/pgmj.22.246.118 on 1 April 1946. Downloaded from 118 POST-GRADUATE MEDICAL JOURNAL April, I946 If the patient is allowed fluid by mouth, it 4. If the apparatus does not appear to be work- should be given by the feeder between times of ing, the clips should be clamped, the appa- aspiration. ratus disconnected from the gastric tube and Aspiration is performed at regular intervals some water injected down the tube to make either every hour or half-hour according to sure that it is patent. instructions. The aspirated material must always be saved The Time for Removal of the Tube: The tube is for inspection. removed under the Doctor's instructions. In 2. Continuous Suction: A study of the diagram cases of intestinal obstruction this is usually (Fig. 2) will explain the work of the apparatus, after the patient has had one bowel action, and and attention must be to the has passed flatus on at least two occasions. particular paid After operations on the stomach, the tube is following points:- removed when the aspirated contents appear I. The reservoir A must never be allowed to clear contain bile. become empty. and 2. The end of the tubing in bottle B must be Vomiting: If a patient who is undergoing gastro- below the level of the water. intestinal suction vomits, it indicates that there 3. The clips D and E must be clamped before is some fault in the procedure, and is a reflection and during the changing of the bottles A and on the management of the suction. -

Federal Register/Vol. 71, No. 45/Wednesday, March 8
Federal Register / Vol. 71, No. 45 / Wednesday, March 8, 2006 / Proposed Rules 11563 MAJOR MILESTONES IN LEAD NAAQS REVIEW—Continued Major milestones Completed/future target date(s) Second Draft SP and Second Draft Human Health and Ecological Risk Assessment Reports for Mid-June 2007. CASAC and Public Comment. CASAC Meeting on Second Draft SP and Second Draft Human Health and Ecological Risk Assess- Late July 2007. ment Reports. Complete Final SP and Final Human Health and Ecological Risk Assessment Reports ....................... Late September 2007. Publish Proposal Notice in FEDERAL REGISTER ........................................................................................ Late February 2008. Final Promulgation Notice Signed by Administrator ................................................................................ September 1, 2008. List of Subjects in 40 CFR Part 50 Corporation, and the thermal coal dryers ENVIRONMENTAL PROTECTION Environmental protection, Air at EME Homer City, LP. These three AGENCY pollution control, Carbon monoxide, formerly RACT-subject sources have Lead, Nitrogen dioxide, Ozone, been permanently shut down and the 40 CFR Part 180 Particulate matter, Sulfur oxides. Pennsylvania DEP has indicated to EPA [EPA–HQ–OPP–2005–0325; FRL–7750–8] Dated: February 23, 2006. that no RACT need be approved for them. Ethylenediaminetetraacetic Acid Jeffrey S. Clark, Chemicals: Exemptions from the Acting Director, Office of Air Quality Planning DATES: Effective Date: The proposed rule Requirement of a Tolerance and Standards. for Doverspike Brothers Coal Co., [FR Doc. E6–3225 Filed 3–7–06; 8:45 am] Hedstrom Corporation, and the thermal AGENCY: Environmental Protection BILLING CODE 6560–50–P coal dryers at EME Homer City Agency (EPA). published at 65 FR 20788 is withdrawn ACTION: Proposed rule. ENVIRONMENTAL PROTECTION as of March 8, 2006. -
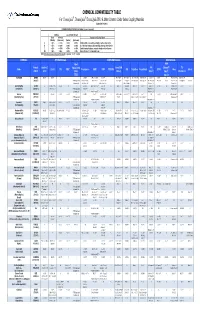
CPC Chemical Compatibility Chart
CHEMICAL COMPATIBILITY TABLE For ChemQuik®, DrumQuik®, DrumQuik PRO & Other Common Colder Series Coupling Materials (Updated 01/14/2010) INTERPRETATION OF TEST DATA (In 30 days to 1 year of exposure) Swelling Loss of Tensile Strength Linear Volumetric Description of Chemical Attack (Plastics) (Elastomers) (Plastics) (Elastomers) A < 10% <= 15% < 15% <=15% Excellent, little or no swelling, softening or surface deterioration B < 15% <= 30% < 30% <= 30% Good chemical resistance, minor swelling, softening or deterioration C < 20% <= 50% < 50% <= 60% Limited chemical resistance, moderate attack, conditional service NR > 20% > 50% > 50% > 60% Severe attack, not recommended for use NOTE: All temperatures are in degrees Fahrenheit. Conversion: °C = (°F - 32)/1.8 CHEMICAL SPRING Materials COUPLING Materials SEAL Materials Teflon® FFKM ® Formula Hastelloy C Encapsulated Acetal/POM FKM (Chemraz / TPO Name 316 SS PPS PEEK™ Polypropylene HDPE PVDF PTFE/PFA ABS Polysulfone Polycarbonate EPDM Buna Silicone (CAS #) (276) 316SS (Celcon) (Viton®) Simriz® / (Santoprene) (TESS) Kalrez®) Acetic Acid C2H4O2 A to 212° 212° A to 212° 212° A A A A to 140° 140° AB to 100% to 70° 70° A to 122° 122° A AAto5%to70 to 5% to 70°° AB 10% to 70° 70° A to 100% to 70° 70° A to 50% to 70° 70° A 10% to 70° 70° AAto70 to 70°° A B to 30% at 70° 70° A to 30% to 70° 70° A (64-19-7) (PTFE Encapsulated AB 50-100% to 160° AB 60% to 180° A to 10% to 225° BC 10% @ 70° C 20% @ 70° A to 20% to 140° B to 50% @ 122° B 10-25% to 100° AB to 200° A to 70° B to 20% to 185° C 50% @ 70° A to