The Plant Disease Clinic and Weed Identification Lab Annual Report 2016
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
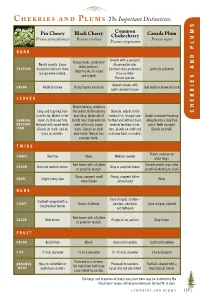
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

Kentucky Fruit Facts
Kentucky Fruit Facts January-February Newsletter 2018 http://www.uky.edu/hort/documents-list-fruit-facts Cooperative Extension Service John Strang, Extension Fruit Specialist, Editor University of Kentucky Horticulture Department Denise Stephens, Newsletter Designer 1100 So. Limestone St. Lexington Ky 40546-0091 (859) 257-2909 Inside this Issue: Fax: (859) 257-2859 Fruit Crop News . .1 extension.ca.uky.edu Upcoming Meetings. .2 Thornless Erect Blackberry Cultivar Trial . 3 of the state showing that we had a few spots that Pesticide Certification: Who Needs It? . .4 were particularly cold and peach flower bud damage Black Knot. .6 probably occurred in these areas. The temperatures Cane Diseases of Brambles. .6 below 0°F more than likely caused damage to Receiving Fruit Facts on the Internet . .8 thornless blackberries. The rule of thumb is that for every degree below 0°F thornless blackberries lose 10% of the crop. Keep in mind that these Fruit Crop News low temperatures occurred in early January when John Strang, U.K. Extension Horticulturist and Matt Dixon, blackberries were at their maximum hardiness level U.K. Ag Meteorologist so there may not be any injury to plants where the temperature was a few degrees below 0°F. We are hopefully through the coldest portion While pruning watch for signs of San Jose of the winter and it is time to start pruning apple and Scale in your trees. Feeding by each insect causes pear trees. Prune the oldest trees first leaving your an increase in the reddish purple pigments beneath youngest until later in the spring. If you have semi- the bark leaving a small circular spot around the dwarf or dwarf trees that are spaced out and trained to feeding site. -

Propagation of Sand Plum (Prunus Angustifolia) Marsh.: an Exciting Start to Domestication
PROPAGATION OF SAND PLUM (PRUNUS ANGUSTIFOLIA) MARSH.: AN EXCITING START TO DOMESTICATION By ELIZABETH ANN MCMAHON Bachelor of Science in Rangeland Management and Horticulture Texas A&M University College Station, TX 2010 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE HORTICULTURE July, 2013 ii PROPAGATION OF SAND PLUM (PRUNUS ANGUSTIFOLIA) MARSH.: AN EXCITING START TO DOMESTICATION Thesis Approved: Dr. Bruce Dunn Thesis Adviser Dr. Eric Stafne Dr. Karen R. Hickman ii ACKNOWLEDGMENTS Funding for this grant was provided by the Oklahoma Specialty Crop Research grant through the Oklahoma Department of Agriculture, Food, and Forestry. I would sincerely like to thank them for awarding this grant to my professors, and for giving me the chance to work on this project. I would like to thank Dr. Bruce Dunn for the time that he spent assisting me with my thesis, as well as his advice on my research. Additional thanks are owed to Dr. Karen Hickmann and Dr. Eric Stafne for agreeing to be on my committee and for helping me with abstracts. Thank you to my fellow graduate students: Li Jiang, for your company when I was working on my proposal; Rania, for your help and being someone to talk to about graduate school; and Stephen, for watering my practice grafts and helping me plant at Perkins. Thank you to Leon and Eddy for the use of their land as field plots and a special thanks to Leon for his advice on the sand plums as well as his eagerness to try different graft techniques. -

The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae)
The Great Lakes Entomologist Volume 25 Number 3 - Fall 1992 Number 3 - Fall 1992 Article 2 October 1992 The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae) David J. Biddinger Pennsylvania State University Angus J. Howitt Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Biddinger, David J. and Howitt, Angus J. 1992. "The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae)," The Great Lakes Entomologist, vol 25 (3) Available at: https://scholar.valpo.edu/tgle/vol25/iss3/2 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Biddinger and Howitt: The Food Plants and Distribution of the American Plum Borer (Lepi 1992 THE GREAT LAKES ENTOMOlOGIST 149 THE FOOD PLANTS AND DISTRIBUTION OF THE AMERICAN PLUM BORER (LEPIDOPTERA: PYRALlDAE)l David J. Biddinger2 and Angus J. Howitt3 ABSTRACT The North American geographical and host plant distributions for the American plnm borer, Euzophera semifuneralis, are reported. Literature and curatorial surveys found the plum borer to be present in 34 states in the U. S. as well as parts of Canada, Mexico, and South America. Pheromone surveys and direct observation found it to be present in high numbers in most cherry and plum orchards in Michigan and in 28 counties of the lower peninsula. A very wide host range representing 15 plant families was found, with most host species in the Rosaceae. -

US EPA, Pesticide Product Label, TETRASUL 4S5,11/23/2015
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION November 23, 2015 OR-CAL, Inc. c/o Molly E. Hickman Agent for OR-CAL, Inc. Hickman Regulatory Services 19699 Mountaineer Way E123 Bend, OR 97702 Subject: Label Amendment – Adding label language and use sites from Rex Lime Sulfur Solution (EPA Reg. No. 71096-6); additional package sizes (space kraft 110 Gall and 220 Gal containers); adding slightly modified first add/precautionary referral statement at the request of New York state; adding the K+ logo (from Rex Lime Sulfur) as an optional marketing statement Product Name: Tetrasul 4S5 EPA Registration Number: 71096-11 Application Date: 7/9/14; Resubmission Dates: 9/9/14; 1/20/15; 11/9/15 Decision Number: 493613 Dear Ms. Hickman: The amended label referred to above, submitted in connection with registration under the Federal Insecticide, Fungicide and Rodenticide Act, as amended, is acceptable. This approval does not affect any conditions that were previously imposed on this registration. You continue to be subject to existing conditions on your registration and any deadlines connected with them. A stamped copy of your labeling is enclosed for your records. This labeling supersedes all previously accepted labeling. You must submit one copy of the final printed labeling before you release the product for shipment with the new labeling. In accordance with 40 CFR 152.130(c), you may distribute or sell this product under the previously approved labeling for 18 months from the date of this letter. After 18 months, you may only distribute or sell this product if it bears this new revised labeling or subsequently approved labeling. -

Proposed Generic Names for Dothideomycetes
Naming and outline of Dothideomycetes–2014 Nalin N. Wijayawardene1, 2, Pedro W. Crous3, Paul M. Kirk4, David L. Hawksworth4, 5, 6, Dongqin Dai1, 2, Eric Boehm7, Saranyaphat Boonmee1, 2, Uwe Braun8, Putarak Chomnunti1, 2, , Melvina J. D'souza1, 2, Paul Diederich9, Asha Dissanayake1, 2, 10, Mingkhuan Doilom1, 2, Francesco Doveri11, Singang Hongsanan1, 2, E.B. Gareth Jones12, 13, Johannes Z. Groenewald3, Ruvishika Jayawardena1, 2, 10, James D. Lawrey14, Yan Mei Li15, 16, Yong Xiang Liu17, Robert Lücking18, Hugo Madrid3, Dimuthu S. Manamgoda1, 2, Jutamart Monkai1, 2, Lucia Muggia19, 20, Matthew P. Nelsen18, 21, Ka-Lai Pang22, Rungtiwa Phookamsak1, 2, Indunil Senanayake1, 2, Carol A. Shearer23, Satinee Suetrong24, Kazuaki Tanaka25, Kasun M. Thambugala1, 2, 17, Saowanee Wikee1, 2, Hai-Xia Wu15, 16, Ying Zhang26, Begoña Aguirre-Hudson5, Siti A. Alias27, André Aptroot28, Ali H. Bahkali29, Jose L. Bezerra30, Jayarama D. Bhat1, 2, 31, Ekachai Chukeatirote1, 2, Cécile Gueidan5, Kazuyuki Hirayama25, G. Sybren De Hoog3, Ji Chuan Kang32, Kerry Knudsen33, Wen Jing Li1, 2, Xinghong Li10, ZouYi Liu17, Ausana Mapook1, 2, Eric H.C. McKenzie34, Andrew N. Miller35, Peter E. Mortimer36, 37, Dhanushka Nadeeshan1, 2, Alan J.L. Phillips38, Huzefa A. Raja39, Christian Scheuer19, Felix Schumm40, Joanne E. Taylor41, Qing Tian1, 2, Saowaluck Tibpromma1, 2, Yong Wang42, Jianchu Xu3, 4, Jiye Yan10, Supalak Yacharoen1, 2, Min Zhang15, 16, Joyce Woudenberg3 and K. D. Hyde1, 2, 37, 38 1Institute of Excellence in Fungal Research and 2School of Science, Mae Fah Luang University, -

Prunus Prunus Prunus Is a Large Genus of Over 400 Species and Hybrids in the Rose Family Which Includes Apri Cots, Nectarines, Peaches, Cherries, Plums and Almonds
nysipm.cornell.edu 2019 Search for this title at the NYSIPM Publications collection: ecommons.cornell.edu/handle/1813/41246 Disease and Insect Resistant Ornamental Plants Mary Thurn, Elizabeth Lamb, and Brian Eshenaur New York State Integrated Pest Management Program, Cornell University Prunus Prunus Prunus is a large genus of over 400 species and hybrids in the rose family which includes apri cots, nectarines, peaches, cherries, plums and almonds. Stone fruit trees are important agri cultural crops, but many are also grown for their ornamental value. Flowering cherries are prized for their showy spring blooms, fall foliage and attractive bark. The historic Tidal Basin cherry trees in Wash ington, DC are perhaps the most famous display of this popular landscape plant in the US. Flow ering plums, particularly purple-leaved cultivars, are also widely grown. Like other members of the rose family, Prunus has many potential disease and insect prob lems and some types may be short-lived in the landscape. DISEASES Bacterial Canker caused by Pseudomonas syringae pv. syringae affects many plants, including ornamental cherries and plums. It is a common problem in parts of the Pacific Northwest where cool, wet spring weather is prevalent (15). On Prunus, the disease causes blossom blight, necrotic spots on leaves and fruit, and branch cankers. Prunus sargentii ‘Rancho' and P. x yedoensis ‘Akebono' appear to have some resistance (16). Black Knot is a fungal disease caused by Apiosporina morbosa (syn. Dibotryon morbosum) that affects both wild and cultivated species in the genus Prunus, especially plums and cherries. Infected branches develop unsightly elongated swellings or galls that can girdle branches, causing decline and dieback. -

The Venturiaceae in North America: Revisions and Additions
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 1989 Band/Volume: 41 Autor(en)/Author(s): Barr Margaret E. Artikel/Article: The Venturiaceae in North America: Revisions and Additions. 25-40 ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at The Venturiaceae in North America: Revisions and Additions M. E. BARR Department of Botany, University of Massachussets, Amherst, MA, 01003 USA BARR, M. (1989). The Venturiaceae in North America: Revisions and Additions. - SYDOWIA 41: 25-40. The Venturiaceae (Pleosporales) in North America is revised to include Ven- turia, Platychora, Coleroa, Pyrenobotrys, Phaeocryptopus, Dibotryon, Xenomeris, Gibbera, Apiosporina, Metacoleroa, Acantharia, and Protoventuria. Notes and re- vised keys to species of the genera are provided. Venturia iridicola, Protoventuria angustispora, and P. arizonica are described as new. New combinations are proposed for Coleroa concinna, Plowrightia abietis, Protoventuria cassandrae, P. myrtilli, P. gaultheriae, P. kalmiae, P. andromedae, P. ramicola, P. major, and P. elegantula. The concept of the family Venturiaceae (Pleosporales) in North America has been revised and limited (BARR, 1987b) from that of BARR (1968). The family is more restricted than its circumscription by MÜLLER & VON ARX (1962), LUTTRELL (1973), VON ARX & MÜLLER (1975), O. ERIKSSON & HAWKSWORTH (1987). Studies by a number of authors, e. g., B. ERIKSSON (1974), SIVANESAN (1977), and MORELET (1983) have resulted in additions to taxa or name changes that are accepted in the family. It seems time to provide a revised key to genera of the family, keys to species of the genera, and additional notes on some taxa. -

Black Knot Nicole Ward Gauthier Dennis Morgeson Extension Plant Pathologist County Extension Agent
University of Kentucky College of Agriculture Plant Pathology Extension CooPeRative exTension SeRvice UnIversity oF Kentucky College oF agriculture, FooD anD environmenT Plant Pathology Fact Sheet PPFS-FR-T-04 Black Knot Nicole Ward Gauthier Dennis Morgeson Extension Plant Pathologist County Extension Agent Introduction Cause & Disease Development Black knot is a common, often serious, disease of Black knot is caused by the fungus Apiosporina plums and cherries in Kentucky. Ornamental Prunus morbosa (syn. Dibotryon morbosum), which species, as well as wild plums and cherries, may also overwinters in knots on previously infected twigs be affected. Trees in both commercial and residential and branches. Spores develop within knots in plantings are susceptible. spring between bud break and shuck split and are spread by wind and rain. Only elongating (actively Symptoms growing) twigs of the current season’s growth are susceptible. While infection takes place in spring, Black knot is aptly named for the conspicuous black knot development is not evident until autumn. knotty growths that form on infected branches (Figure 1). Initially, however, these irregular swellings or knots are small and light brown (Figure 2). One year after infection, the enlarging knots become olive-green with a velvety surface. As the season progresses, swellings harden, become brittle, and turn black in color (Figure 3), reaching lengths of 6 inches by the end of the growing season. Often only one side of a limb is affected (Figure 4); but, in some cases, limbs may become completely encircled. Knots continue to expand each year until girdled branches eventually die. FIGURE 1. BLACK KNOT DISEASE IS EASILY RECOGNIZED BY THE BLACK KNOTTY GROWTHS ON TWIGS AND BRANCHES.