The Venturiaceae in North America: Revisions and Additions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schlechtendalia 37 (2020)
Schlechtendalia 38, 2021 Annotated list of taxonomic novelties published in “Fungi Europaei Exsiccati, Klotzschii Herbarium Vivum Mycologicum Continuato, Editio Nova, Series Secunda” Cent. 1 to 26 issued by G. L. Rabenhorst between 1859 and 1881 (second part – Cent. 11 to 20) Uwe BRAUN & Konstanze BENSCH Abstract: Braun, U. & Bensch, K. 2021: Annotated list of taxonomic novelties published in “Fungi Europaei Exsiccati, Klotzschii Herbarium Vivum Mycologicum Continuato, Editio Nova, Series Secunda” Cent. 1 to 26 issued by G. L. Rabenhorst between 1859 and 1881 (second part – Cent. 11 to 20). Schlechtendalia 38: 191–262. New taxa and new combinations published by G. L. Rabenhorst in “Fungi Europaei Exsiccati, Klotzschii Herbarium Vivum Mycologicum, Editio Nova, Series Secunda” Cent. 1 to 26 in the second half of the 19th century are listed and annotated. References, citations and the synonymy are corrected when necessary. The nomenclature of some taxa is discussed in more detail. The second part of this treatment comprises taxonomic novelties in Cent. 11 to 20. Zusammenfassung: Braun, U. & Bensch, K. 2021: Kommentierte Liste taxonomischer Neuheiten publiziert in „Fungi Europaei Exsiccati, Klotzschii Herbarium Vivum Mycologicum Continuato, Editio Nova, Series Secunda“ Cent. 1 bis 26, herausgegeben von G. L. Rabenhorst zwischen 1859 und 1881 (zweiter Teil, Cent. 11 bis 20). Schlechtendalia 38: 191–262. Neue Taxa und Kombinationen publiziert von G. L. Rabenhorst in “Klotzschii Herbarium Vivum Mycologicum, Editio Nova” Cent. 1 bis 26 in der zweiten Hälfte des 19. Jahrhunderts werden aufgelistet und annotiert. Referenzangaben, Zitate und die Synonymie werden korrigiert falls notwendig. Die Nomenklatur einiger Taxa wird detaillierter besprochen. Der zweite Teil dieser Bearbeitung umfasst Cent. -

Venturia Inaequalis (Cooke) Winter, Hedwigia 36: 81, 1897
Set No 41, published 1974 CMI Descriptions of VENTURIA INAEQUALIS Pathogenic Fungi and Bacteria No. 401 Venturia inaequalis (Cooke) Winter, Hedwigia 36: 81, 1897. Sphaerella inaequalis Cooke, 1866. Spilosticta inaequalis (Cooke) Petr., 1940. Endostigma inaequalis (Cooke) Syd., 1923. Sphaeria cinerascens Fuckel, 1863. Sphaerella cinerascens Fuckel, 1870. Conidial state: Spilocaea pomi Fr., 1825. Fusicladium pomi (Fr.) Lind, 1913. Helminthosporium pyrorum Lib. (pro parte), 1832. (For further synonymy see Barr, Canadian Journal of Botany 46: 808, 1968 and Hughes, Canadian Journal of Botany 31: 566–568, 1953.) Pseudothecia immersed, globose, amphigenous, scattered or grouped, with or without setae. Asci cylindrical, bitunicate, 8- spored, 60–70 × 7–12 µm. Ascospores monostichous or distichous, olivaceous brown, septate in the upper third, with upper ends tapering and lower ends rounded, 12–15 × 6–8 µm. Conidial state: Conidiophores arising from subcuticular or intraepidermal mycelium which forms radiating plates, simple cylindrical, pale to mid brown to olivaceous brown, sometimes swollen at the base, variable in length, up to 90 µm long, 5–6 µm wide. Stroma often formed as pseudoparenchyma. Conidia produced singly at the tip of the conidiophore and then successively by proliferation through scars of the detached conidia, resulting in characteristic and distinct annellations on the conidiophores; obpyriform to obclavate, pale to mid olivaceous brown, smooth, 0–1-septate, 12–30 µm long, 6–10 µm wide in the broadest part with a truncate base 4–5 µm wide. © CAB INTERNATIONAL 1998 Set No 41, published 1974 HOSTS: Principally on apple (Malus pumila), and other species of Malus. Also recorded on Pyrus spp., Sorbus spp. -

Caracterização De Fungos Cercospóroides Associados À Vegetação De Mata Atlântica E Cercanias, No Estado Do Rio De Janeiro
UFRRJ INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA DISSERTAÇÃO Caracterização de Fungos Cercospóroides Associados à Vegetação de Mata Atlântica e Cercanias, no Estado do Rio de Janeiro Kerly Martínez Andrade 2016 UNIVERSIDADE FEDERAL RURAL DO RIO DE JANEIRO INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA CARACTERIZAÇÃO DE FUNGOS CERCOSPORÓIDES ASSOCIADOS À VEGETAÇÃO DE MATA ATLÂNTICA E CERCANIAS NO ESTADO DO RIO DE JANEIRO KERLY MARTÍNEZ ANDRADE Sob a Orientação do Professor Dr. Carlos Antonio Inácio Dissertação submetida como requisito parcial para obtenção do grau de Mestre em Ciências, no Programa de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada, Área de Concentração em Fitossanidade. Seropédica, RJ Agosto, 2016 i UFRRJ / Biblioteca Central / Divisão de Processamentos Técnicos 579.5 A553c Andrade, Kerly Martínez, 1989- T Caracterização de fungos cercosporóides associados à vegetação de Mata Atlântica e cercanias no Estado do Rio de Janeiro / Kerly Martínez Andrade. – 2016. 136 f.ail. Orientador: Carlos Antonio Inácio. Dissertação (mestrado) – Universidade Federal Rural do Rio de Janeiro, Curso de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada, 2016. Bibliografia: f. 114-121. 1. Fungos - Teses. 2. Fungos - Morfologia - Teses. 3. Cercospora - Teses. 4. Fungos fitopatogênicos – Mata Atlântica – Teses. 5. Plantas – Parasito – Mata Atlântica – Teses. I. Inácio, Carlos Antonio, 1966- II. Universidade Federal Rural do Rio de Janeiro. Curso de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada. III. Título. ii UNIVERSIDADE FEDERAL RURAL DO RIO DE JANEIRO INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA KERLY MARTÍNEZ ANDRADE Dissertação submetida como requisito parcial para obtenção do grau de Mestre em Ciências, no Programa de Pós-Graduação em Fitosanidade e Biotecnologia Aplicada, Área de Concentração em Fitossanidade. -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
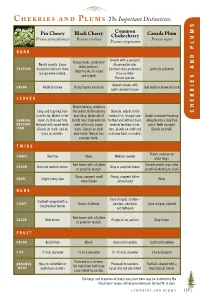
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

The Genus Fusicladium (Hyphomycetes) in Poland
ACTA MYCOLOGICA Dedicated to Professor Alina Skirgiełło Vol. 41 (2): 285-298 on the occasion of her ninety-fifth birthday 2006 The genus Fusicladium (Hyphomycetes) in Poland MAŁGORZATA RUSZKIEWICZ-MICHALSKA and EWA POŁEĆ 1 Department of Algology and Mycology, University of Łódź, Banacha 12/16, PL-90-237 Łódź [email protected]; [email protected] Ruszkiewicz-Michalska M., Połeć E.: The genus Fusicladium (Hyphomycetes) in Poland. Acta Mycol. 41 (2): 285-298, 2006. The paper presents new and historical data on the genus Fusicladium verified on the base of the recently published critical monograph. Fifteen species recorded in Poland under the name Fusicladium and synonymous Pollaccia and Spilocaea are reported; 5 are documented by authors’ materials from Central Poland while the other taxa are supported with literature data only, including three species belonging currently to Fusicladiella and Passalora. Three species, reported here for the first time in Poland: Fusicladium convolvularum Ondřej, F. scribnerianum (Cavara) M. B. Ellis and F. virgaureae Ondřej, are known from a few localities in the world. All the species are provided with the distribution maps and the newly reported ones are illustrated with ink drawings. Key words: parasitic fungi, anamorphic fungi, Deuteromycotina, distribution, Poland INTRODUCTION Worldwide 57 fungal taxa belong to the anamorphic genus Fusicladium Bonord. em. Schubert, Ritschel et U. Braun. They are phytopathologically relevant patho- gens, causing leaf spots, necroses, scab diseases as well as leaf and fruit deformations of members of at least 52 angiospermous plant genera (Schubert, Ritschel, Braun 2003). The fungi are host specific, mostly confined to a single host genus or allied host genera in a single family, e.g. -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

<I>Tothia Fuscella</I>
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/118.203 Volume 118, pp. 203–211 October–December 2011 Epitypification, morphology, and phylogeny of Tothia fuscella Haixia Wu1, Walter M. Jaklitsch2, Hermann Voglmayr2 & Kevin D. Hyde1, 3, 4* 1 International Fungal Research and Development Centre, Key Laboratory of Resource Insect Cultivation & Utilization, State Forestry Administration, The Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, 650224, PR China 2 Department of Systematic and Evolutionary Botany, Faculty Centre of Biodiversity, University of Vienna, Rennweg 14, A-1030 Wien, Austria 3 School of Science, Mae Fah Luang University, Tasud, Muang, Chiang Rai 57100, Thailand 4 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 11442, Saudi Arabia *Correspondence to: [email protected] Abstract — The holotype of Tothia fuscella has been re-examined and is re-described and illustrated. An identical fresh specimen from Austria is used to designate an epitype with herbarium material and a living culture. Sequence analyses show T. fuscella to be most closely related to Venturiaceae and not Microthyriaceae, to which it was previously referred. Key words — Dothideomycetes, molecular phylogeny, taxonomy Introduction We have been re-describing and illustrating the generic types of Dothideomycetes (Zhang et al. 2008, 2009, Wu et al. 2010, 2011, Li et al. 2011) and have tried where possible to obtain fresh specimens for epitypification and use molecular analyses to provide a natural classification. Our previous studies of genera in the Microthyriaceae, a poorly known family within the Dothideomycetes, have resulted in several advances (Wu et al. -

Fungal Cannons: Explosive Spore Discharge in the Ascomycota Frances Trail
MINIREVIEW Fungal cannons: explosive spore discharge in the Ascomycota Frances Trail Department of Plant Biology and Department of Plant Pathology, Michigan State University, East Lansing, MI, USA Correspondence: Frances Trail, Department Abstract Downloaded from https://academic.oup.com/femsle/article/276/1/12/593867 by guest on 24 September 2021 of Plant Biology, Michigan State University, East Lansing, MI 48824, USA. Tel.: 11 517 The ascomycetous fungi produce prodigious amounts of spores through both 432 2939; fax: 11 517 353 1926; asexual and sexual reproduction. Their sexual spores (ascospores) develop within e-mail: [email protected] tubular sacs called asci that act as small water cannons and expel the spores into the air. Dispersal of spores by forcible discharge is important for dissemination of Received 15 June 2007; revised 28 July 2007; many fungal plant diseases and for the dispersal of many saprophytic fungi. The accepted 30 July 2007. mechanism has long been thought to be driven by turgor pressure within the First published online 3 September 2007. extending ascus; however, relatively little genetic and physiological work has been carried out on the mechanism. Recent studies have measured the pressures within DOI:10.1111/j.1574-6968.2007.00900.x the ascus and quantified the components of the ascus epiplasmic fluid that contribute to the osmotic potential. Few species have been examined in detail, Editor: Richard Staples but the results indicate diversity in ascus function that reflects ascus size, fruiting Keywords body type, and the niche of the particular species. ascus; ascospore; turgor pressure; perithecium; apothecium. 2 and 3). Each subphylum contains members that forcibly Introduction discharge their spores. -

Selection and Orchard Testing of Antagonists Suppressing Conidial Production by the Apple Scab Pathogen Venturia Inaequalis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wageningen University & Research Publications Eur J Plant Pathol DOI 10.1007/s10658-008-9377-z Selection and orchard testing of antagonists suppressing conidial production by the apple scab pathogen Venturia inaequalis Jürgen J. Köhl & Wilma W. M. L. Molhoek & Belia B. H. Groenenboom-de Haas & Helen H. M. Goossen-van de Geijn Received: 7 April 2008 /Accepted: 29 September 2008 # KNPV 2008 Abstract Apple scab caused by Venturia inaequalis concluded that C. cladosporioides H39 has promising is a major disease in apple production. Epidemics in potential as a biological control agent for apple scab spring are initiated by ascospores produced on over- control. More information is needed on the effect of C. wintering leaves whereas epidemics during summer cladosporioides H39onapplescabepidemicsaswellas are driven by conidia produced on apple leaves by on mass production, formulation and shelf life of biotrophic mycelium. Fungal colonisers of sporulating conidia of the antagonist. colonies of V. inaequalis were isolated and their potential to reduce the production of conidia of V. Keywords Antagonist screening . Biological control . inaequalis was evaluated on apple seedlings under Sporulation controlled conditions. The four most effective isolates of the 63 screened isolates were tested subsequently under Dutch orchard conditions in 2006. Repeated Introduction applications of conidial suspensions of Cladosporium cladosporioides H39 resulted in an average reduction of Apple scab, caused by Venturia inaequalis is a major conidial production by V. inaequalis of approximately disease in world-wide apple production (MacHardy 40%. In 2007, applications of conidial suspensions of C. -

Determination of the Genetic Structure of Venturia Inaequalis Populations by Means of Molecular Markers
SSis. EW <*-* -"B Diss. ETHNr. 12767 Determination of the Genetic Structure of Venturia inaequalis Populations by Means of Molecular Markers A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences presented by CatE Isabel Tenzer Dipl. sc. nat. ETH born January 8th, 1971 citizen of Germany accepted on the recommendation of Prof. Dr. B. A. Roy, examiner Dr. C. Gessler, co-examiner Prof. Dr. G. Defago, co-examiner 1998 1 Contents Contents Page Abstract 2 zusammenfassung 3 Introduction 5 Chapter 1 15 Subdivision and genetic structure of four populations of Venturia inaequalis in Switzerland Chapter 2 28 Genetic diversity of Venturia inaequalis across Europe Chapter 3 41 Identification of microsatellite markers and their application to population genetics of Venturia inaequalis Discussion 57 Curriculum vitae 68 Verdankungen 69 2 Abstract Abstract Venturia inaequalis is the causal agent of apple scab, the most important disease in apple production in Europe. The pathogen is mainly controlled by fungicides, but the plantation of scab-resistant apple cultivars becomes increasingly important, being a more ecological cultivation strategy. The mostly used resistance gene (Vf) originated from Malus floribunda 821, but a break down of this resistance has already been reported in 1984 in Ahrensburg (Northern Germany), in 1994 in Kent (Great Britain), and in 1997 in Wilheminadorp (The Netherlands). Since most new resistant varieties carry the V/-resistance, concern about a large scale breakdown of this resistance increases. However, the V/-resistance is still largely effective despite of growing Vf-resistant cultivars in European as well as in American breeding stations for more than 30 years and for a shorter time in commercial orchards.