Propagation of Sand Plum (Prunus Angustifolia) Marsh.: an Exciting Start to Domestication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Montgomery County Landscape Plant List
9020 Airport Road Conroe, TX 77303 (936) 539-7824 MONTGOMERY COUNTY LANDSCAPE PLANT LIST Scientific Name Common Name Size Habit Light Water Native Wildlife Comments PERENNIALS Abelmoschus ‘Oriental Red’ Hibiscus, Oriental Red 3 x 3 D F L N Root hardy, reseeds Abutilon sp. Flowering Maple Var D F M N Acalypha pendula Firetail Chenille 8" x 8" E P H N Acanthus mollis Bear's Breeches 3 x 3 D S M N Root hardy Acorus gramineus Sweet Flag 1 x 1 E P M N Achillea millefolium var. rosea Yarrow, Pink 2 x 2 E F/P M N BF Butterfly nectar plant Adiantum capillus-veneris Fern, Maidenhair 1 x 1 E P/S H Y Dormant when dry Adiantum hispidulum Fern, Rosy Maidenhair 1 x 1 D S H N Agapanthus africanus Lily of the Nile 2 x 2 E P M N Agastache ‘Black Adder’ Agastache, Black Adder 2 x 2 D F M N BF, HB Butterfly/hummingbird nectar plant Ageratina havanensis Mistflower, Fragrant 3 x 3 D F/P L Y BF Can take poor drainage Ageratina wrightii Mistflower, White 2 x 2 D F/P L Y BF Butterfly nectar plant Ajuga reptans Bugle Flower 6" x 6" E P/S M N Alocasia sp. Taro Var D P M N Aggressive in wet areas Aloysia virgata Almond Verbena 8 x 5 D S L N BF Very fragrant, nectar plant Alpinia sp. Gingers, Shell 6 x 6 E F/P M N Amsonia tabernaemontana Texas Blue Star 3 x 3 D P M Y Can take poor drainage Andropogon gerardii Bluestem, Big 3 to 8 D F/P L Y Andropogon glomeratus Bluestem, Brushy 2 to 5 D F/P L Y Andropogon ternarius Bluestem, Splitbeard 1 to 4 D F/P L Y Anisacanthus wrightii Flame Acanthus 3 x 3 D F L Y HB Hummingbird nectar plant Aquilegia chrysantha Columbine, Yellow 2 x 1 E P/S M Y Dormant when dry, reseeds Aquilegia canadensis Columbine, Red 1 x 1 E P/S M Y Dormant when dry, reseeds Ardisia crenata Ardisia 1 x 1 E P/S M N Ardisia japonica Ardisia 2 x 2 E P/S M N Artemisia sp. -

Plum Crazy: Rediscovering Our Lost Prunus Resources W.R
Plum Crazy: Rediscovering Our Lost Prunus Resources W.R. Okie1 U.S. Department of Agriculture–Agricultural Research Service, Southeastern Fruit and Tree Nut Research Laboratory, 21 Dunbar Road, Byron, GA 31008 Recent utilization of genetic resources of peach [Prunus persica (‘Quetta’ from India, ‘John Rivers’ from England, and ‘Lippiatts’ (L.) Batsch] and Japanese plum (P. salicina Lindl. and hybrids) has from New Zealand) were critical to the development of modern been limited in the United States compared with that of many crops. nectarines in California (Taylor, 1959). However, most fresh-market Difficulties in collection, importation, and quarantine throughput have peach breeding programs in the United States have used germplasm limited the germplasm available. Prunus is more difficult to preserve developed in the United States for cultivar development (Okie, 1998). because more space is needed than for small fruit crops, and the shorter Only in New Jersey was there extensive hybridization with imported life of trees relative to other tree crops because of disease and insect clones, and most of these hybrids have not resulted in named cultivars problems. Lack of suitable rootstocks has also reduced tree life. The (Blake and Edgerton, 1946). trend toward fewer breeding programs, most of which emphasize In recent years, interest in collecting and utilizing novel germplasm “short-term” (long-term compared to most crops) commercial cultivar has increased. For example, non-melting clingstone peaches from development to meet immediate industry needs, has also contributed Mexico and Brazil have been used in the joint USDA–Univ. of to reduced use of exotic material. Georgia–Univ. of Florida breeding program for the development of Probably all modern commercial peaches grown in the United early ripening, non-melting, fresh-market peaches for low-chill areas States are related to ‘Chinese Cling’, a peach imported from China (Beckman and Sherman, 1996). -

Wild Goose Plum Prunus Hortulana ILLINOIS RANGE Tree in Flower
wild goose plum Prunus hortulana Kingdom: Plantae FEATURES Division/Phylum: Magnoliophyta Wild goose plum is a small tree that may attain a Class: Magnoliopsida height of 20 feet and a trunk diameter of eight Order: Rosales inches. Its gray or brown bark becomes scaly at maturity. Twigs are slender, red-brown and smooth. Family: Rosaceae The ovoid, red-brown buds are about one-fourth ILLINOIS STATUS inch in length. Leaves are arranged alternately along the stem. These simple, oblong to oval leaves may common, native be as much as six inches long and two inches wide. Each leaf is finely-toothed along the edges. Each tooth has a gland at its tip. The leaf is green and smooth on the upper surface and pale and sometimes hairy on the lower surface. Flowers develop in clusters, up to one inch wide. The five- petaled, white flowers appear after the leaves are partly grown. The fruit is a drupe (a seed enclosed in a hard, dry material that in turn is covered with a fleshy material). The drupe is nearly spherical and up to one inch in diameter. This red, fleshy fruit is hard, bitter and contains one seed. BEHAVIORS © Guy Sternberg Wild goose plum may be found in the southern one- half of Illinois. It grows in wood edges and thickets. tree in flower Flowers are produced from March through April. The wood is hard and brown. ILLINOIS RANGE © Guy Sternberg flowering branches © Illinois Department of Natural Resources. 2021. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. -

A Checklist of the Vascular Flora of the Mary K. Oxley Nature Center, Tulsa County, Oklahoma
Oklahoma Native Plant Record 29 Volume 13, December 2013 A CHECKLIST OF THE VASCULAR FLORA OF THE MARY K. OXLEY NATURE CENTER, TULSA COUNTY, OKLAHOMA Amy K. Buthod Oklahoma Biological Survey Oklahoma Natural Heritage Inventory Robert Bebb Herbarium University of Oklahoma Norman, OK 73019-0575 (405) 325-4034 Email: [email protected] Keywords: flora, exotics, inventory ABSTRACT This paper reports the results of an inventory of the vascular flora of the Mary K. Oxley Nature Center in Tulsa, Oklahoma. A total of 342 taxa from 75 families and 237 genera were collected from four main vegetation types. The families Asteraceae and Poaceae were the largest, with 49 and 42 taxa, respectively. Fifty-eight exotic taxa were found, representing 17% of the total flora. Twelve taxa tracked by the Oklahoma Natural Heritage Inventory were present. INTRODUCTION clayey sediment (USDA Soil Conservation Service 1977). Climate is Subtropical The objective of this study was to Humid, and summers are humid and warm inventory the vascular plants of the Mary K. with a mean July temperature of 27.5° C Oxley Nature Center (ONC) and to prepare (81.5° F). Winters are mild and short with a a list and voucher specimens for Oxley mean January temperature of 1.5° C personnel to use in education and outreach. (34.7° F) (Trewartha 1968). Mean annual Located within the 1,165.0 ha (2878 ac) precipitation is 106.5 cm (41.929 in), with Mohawk Park in northwestern Tulsa most occurring in the spring and fall County (ONC headquarters located at (Oklahoma Climatological Survey 2013). -

(Prunus Spp) Using Random Amplified Microsatellite Polymorphism Markers
Assessment of genetic diversity and relationships among wild and cultivated Tunisian plums (Prunus spp) using random amplified microsatellite polymorphism markers H. Ben Tamarzizt, S. Ben Mustapha, G. Baraket, D. Abdallah and A. Salhi-Hannachi Laboratory of Molecular Genetics, Immunology & Biotechnology, Faculty of Sciences of Tunis, University of Tunis El Manar, El Manar, Tunis, Tunisia Corresponding author: A. Salhi-Hannachi E-mail: [email protected] Genet. Mol. Res. 14 (1): 1942-1956 (2015) Received January 8, 2014 Accepted July 8, 2014 Published March 20, 2015 DOI http://dx.doi.org/10.4238/2015.March.20.4 ABSTRACT. The usefulness of random amplified microsatellite polymorphism markers to study the genetic diversity and relationships among cultivars belonging to Prunus salicina and P. domestica and their wild relatives (P. insititia and P. spinosa) was investigated. A total of 226 of 234 bands were polymorphic (96.58%). The 226 random amplified microsatellite polymorphism markers were screened using 15 random amplified polymorphic DNA and inter-simple sequence repeat primers combinations for 54 Tunisian plum accessions. The percentage of polymorphic bands (96.58%), the resolving power of primers values (135.70), and the polymorphic information content demonstrated the efficiency of the primers used in this study. The genetic distances between accessions ranged from 0.18 to 0.79 with a mean of 0.24, suggesting a high level of genetic diversity at the intra- and interspecific levels. The unweighted pair group with arithmetic mean dendrogram Genetics and Molecular Research 14 (1): 1942-1956 (2015) ©FUNPEC-RP www.funpecrp.com.br Genetic diversity of Tunisian plums using RAMPO markers 1943 and principal component analysis discriminated cultivars efficiently and illustrated relationships and divergence between spontaneous, locally cultivated, and introduced plum types. -

Plums on the Prairies by Rick Sawatzky
Plums on the Prairies by Rick Sawatzky Information from Literature Much has been published about pollination, pollinators, pollinizers, fertilization and fruit set in text books and periodicals. The definitions are not difficult. Pollination is the movement of pollen among compatible flowering plants (cross-pollination) or from anthers to stigmas on the same plant or different plants of the same clone (self-pollination). Many plants will self-pollinate but set very few fruit; some authors consider them self- pollinating but they are definitely not self-fruitful. Self-fruitful plants (and clones) set a crop of fruit after self-pollination; some of these plants bear fruit with no seeds (parthenocarpy); others develop seeds with embryos that are genetically identical to the parent plant (apomixis); and others produce haploid seeds that develop from an unfertilized egg cell. (When haploid seeds germinate they are very weak seedlings with only half the chromosomes of normal seedlings.) Regarding temperate zone tree fruits, self-pollination and fruit set does not mean self-fertility and the development of normal seeds. Many temperate zone small fruit species (e.g. strawberries and raspberries) are self-fertile and develop maximum yields of fruit with normal seeds as the result of self-pollination by insects. Pollinators, usually insects, are vectors of pollen movement. Pollinizers are plants which provide the appropriate pollen for other plants. Fertilization is the process in which gametes from the pollen unite with egg cells in the ovary of the flower. Normal seeds are usually the result of this process. Also, the principles are easily understood. Poor fertilization in plums and other Prunus species results in a poor fruit set. -
Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -
![Vascular Plants of Williamson County Prunus Rivularis − THICKET PLUM, CREEK PLUM [Rosaceae]](https://docslib.b-cdn.net/cover/2097/vascular-plants-of-williamson-county-prunus-rivularis-thicket-plum-creek-plum-rosaceae-442097.webp)
Vascular Plants of Williamson County Prunus Rivularis − THICKET PLUM, CREEK PLUM [Rosaceae]
Vascular Plants of Williamson County Prunus rivularis − THICKET PLUM, CREEK PLUM [Rosaceae] Prunus rivularis Scheele, THICKET PLUM, CREEK PLUM. Thicket-forming shrub, cloning via shallow, horizontal roots, shoots from spreading roots erect, 200−400 cm tall, in range dense clones to 30 m long; shoots often 2-dimensional (plagiotropic), not spinescent. Stems: somewhat angled, with 3 ridges descending from leaf, zigzagged, internodes to 20 mm long; bark on small branches satiny reddish brown having horizontal lenticels, with scattered stiff short hairs. Leaves: helically alternate, simple, petiolate, with stipules; stipules 2, attached to petiole at top of wing, acuminate, to 7 × < 1 mm, with 1−2 basal lobes or without lobes, having 1−several, fleshy glandular teeth on margins; petiole to 20 mm long, winglike to 3 mm below stipules, above wings strongly channeled, having 1−several glandular teeth along ridges above midpoint, channel stiff-hairy; blade ovate- lanceolate, (15−)50−95+ × (9−)22−35+ mm, tapered at base, low-serrate initially with glandular tips on teeth base-to-tip on margins, acuminate to acute at tip, pinnately veined with principal vein slightly sunken on upper surface and raised on lower surface, often with a pair of glands at base of blade, glands on teeth abscising as blade growing, short-villous aging glabrescent or hairs mostly present along veins on lower surface. Inflorescence: 2−4-flowered cymelike clusters, on lateral shoots < 50 cm long on stubby shoots to 3 mm long, flower-bearing spur shoots covered with bud and bract scars, bracteate; bractlet subtending pedicel fan-shaped, to 3 mm long, early-deciduous; pedicel at anthesis 8−10 mm long not increasing in fruit, glabrous. -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

Winter 2014-2015 (22:3) (PDF)
Contents NATIVE NOTES Page Fern workshop 1-2 Wavey-leaf basket Grass 3 Names Cacalia 4 Trip Report Sandstone Falls 5 Kate’s Mountain Clover* Trip Report Brush Creek Falls 6 Thank yous memorial 7 WEST VIRGINIA NATIVE PLANT SOCIETY NEWSLETTER News of WVNPS 8 VOLUME 22:3 WINTER 2014-15 Events, Dues Form 9 Judy Dumke-Editor: [email protected] Phone 740-894-6859 Magnoliales 10 e e e visit us at www.wvnps.org e e e . Fern Workshop University of Charleston Charleston WV January 17 2015, bad weather date January 24 2015 If you have thought about ferns, looked at them, puzzled over them or just want to know more about them join the WVNPS in Charleston for a workshop led by Mark Watson of the University of Charleston. The session will start at 10 A.M. with a scheduled end point by 12:30 P.M. A board meeting will follow. The sessions will be held in the Clay Tower Building (CTB) room 513, which is the botany lab. If you have any pressed specimens to share, or to ask about, be sure to bring them with as much information as you have on the location and habitat. Even photographs of ferns might be of interest for the session. If you have a hand lens that you favor bring it along as well. DIRECTIONS From the North: Travel I-77 South or 1-79 South into Charleston. Follow the signs to I-64 West. Take Oakwood Road Exit 58A and follow the signs to Route 61 South (MacCorkle Ave.). -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
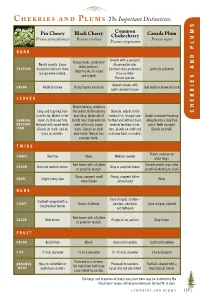
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate,