Research Collection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caracterização De Fungos Cercospóroides Associados À Vegetação De Mata Atlântica E Cercanias, No Estado Do Rio De Janeiro
UFRRJ INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA DISSERTAÇÃO Caracterização de Fungos Cercospóroides Associados à Vegetação de Mata Atlântica e Cercanias, no Estado do Rio de Janeiro Kerly Martínez Andrade 2016 UNIVERSIDADE FEDERAL RURAL DO RIO DE JANEIRO INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA CARACTERIZAÇÃO DE FUNGOS CERCOSPORÓIDES ASSOCIADOS À VEGETAÇÃO DE MATA ATLÂNTICA E CERCANIAS NO ESTADO DO RIO DE JANEIRO KERLY MARTÍNEZ ANDRADE Sob a Orientação do Professor Dr. Carlos Antonio Inácio Dissertação submetida como requisito parcial para obtenção do grau de Mestre em Ciências, no Programa de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada, Área de Concentração em Fitossanidade. Seropédica, RJ Agosto, 2016 i UFRRJ / Biblioteca Central / Divisão de Processamentos Técnicos 579.5 A553c Andrade, Kerly Martínez, 1989- T Caracterização de fungos cercosporóides associados à vegetação de Mata Atlântica e cercanias no Estado do Rio de Janeiro / Kerly Martínez Andrade. – 2016. 136 f.ail. Orientador: Carlos Antonio Inácio. Dissertação (mestrado) – Universidade Federal Rural do Rio de Janeiro, Curso de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada, 2016. Bibliografia: f. 114-121. 1. Fungos - Teses. 2. Fungos - Morfologia - Teses. 3. Cercospora - Teses. 4. Fungos fitopatogênicos – Mata Atlântica – Teses. 5. Plantas – Parasito – Mata Atlântica – Teses. I. Inácio, Carlos Antonio, 1966- II. Universidade Federal Rural do Rio de Janeiro. Curso de Pós-Graduação em Fitossanidade e Biotecnologia Aplicada. III. Título. ii UNIVERSIDADE FEDERAL RURAL DO RIO DE JANEIRO INSTITUTO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE PROGRAMA DE PÓS-GRADUAÇÃO EM FITOSSANIDADE E BIOTECNOLOGIA APLICADA KERLY MARTÍNEZ ANDRADE Dissertação submetida como requisito parcial para obtenção do grau de Mestre em Ciências, no Programa de Pós-Graduação em Fitosanidade e Biotecnologia Aplicada, Área de Concentração em Fitossanidade. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
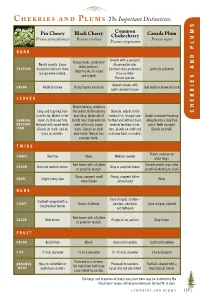
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

<I>Tothia Fuscella</I>
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/118.203 Volume 118, pp. 203–211 October–December 2011 Epitypification, morphology, and phylogeny of Tothia fuscella Haixia Wu1, Walter M. Jaklitsch2, Hermann Voglmayr2 & Kevin D. Hyde1, 3, 4* 1 International Fungal Research and Development Centre, Key Laboratory of Resource Insect Cultivation & Utilization, State Forestry Administration, The Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, 650224, PR China 2 Department of Systematic and Evolutionary Botany, Faculty Centre of Biodiversity, University of Vienna, Rennweg 14, A-1030 Wien, Austria 3 School of Science, Mae Fah Luang University, Tasud, Muang, Chiang Rai 57100, Thailand 4 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 11442, Saudi Arabia *Correspondence to: [email protected] Abstract — The holotype of Tothia fuscella has been re-examined and is re-described and illustrated. An identical fresh specimen from Austria is used to designate an epitype with herbarium material and a living culture. Sequence analyses show T. fuscella to be most closely related to Venturiaceae and not Microthyriaceae, to which it was previously referred. Key words — Dothideomycetes, molecular phylogeny, taxonomy Introduction We have been re-describing and illustrating the generic types of Dothideomycetes (Zhang et al. 2008, 2009, Wu et al. 2010, 2011, Li et al. 2011) and have tried where possible to obtain fresh specimens for epitypification and use molecular analyses to provide a natural classification. Our previous studies of genera in the Microthyriaceae, a poorly known family within the Dothideomycetes, have resulted in several advances (Wu et al. -

Kentucky Fruit Facts
Kentucky Fruit Facts January-February Newsletter 2018 http://www.uky.edu/hort/documents-list-fruit-facts Cooperative Extension Service John Strang, Extension Fruit Specialist, Editor University of Kentucky Horticulture Department Denise Stephens, Newsletter Designer 1100 So. Limestone St. Lexington Ky 40546-0091 (859) 257-2909 Inside this Issue: Fax: (859) 257-2859 Fruit Crop News . .1 extension.ca.uky.edu Upcoming Meetings. .2 Thornless Erect Blackberry Cultivar Trial . 3 of the state showing that we had a few spots that Pesticide Certification: Who Needs It? . .4 were particularly cold and peach flower bud damage Black Knot. .6 probably occurred in these areas. The temperatures Cane Diseases of Brambles. .6 below 0°F more than likely caused damage to Receiving Fruit Facts on the Internet . .8 thornless blackberries. The rule of thumb is that for every degree below 0°F thornless blackberries lose 10% of the crop. Keep in mind that these Fruit Crop News low temperatures occurred in early January when John Strang, U.K. Extension Horticulturist and Matt Dixon, blackberries were at their maximum hardiness level U.K. Ag Meteorologist so there may not be any injury to plants where the temperature was a few degrees below 0°F. We are hopefully through the coldest portion While pruning watch for signs of San Jose of the winter and it is time to start pruning apple and Scale in your trees. Feeding by each insect causes pear trees. Prune the oldest trees first leaving your an increase in the reddish purple pigments beneath youngest until later in the spring. If you have semi- the bark leaving a small circular spot around the dwarf or dwarf trees that are spaced out and trained to feeding site. -

Propagation of Sand Plum (Prunus Angustifolia) Marsh.: an Exciting Start to Domestication
PROPAGATION OF SAND PLUM (PRUNUS ANGUSTIFOLIA) MARSH.: AN EXCITING START TO DOMESTICATION By ELIZABETH ANN MCMAHON Bachelor of Science in Rangeland Management and Horticulture Texas A&M University College Station, TX 2010 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE HORTICULTURE July, 2013 ii PROPAGATION OF SAND PLUM (PRUNUS ANGUSTIFOLIA) MARSH.: AN EXCITING START TO DOMESTICATION Thesis Approved: Dr. Bruce Dunn Thesis Adviser Dr. Eric Stafne Dr. Karen R. Hickman ii ACKNOWLEDGMENTS Funding for this grant was provided by the Oklahoma Specialty Crop Research grant through the Oklahoma Department of Agriculture, Food, and Forestry. I would sincerely like to thank them for awarding this grant to my professors, and for giving me the chance to work on this project. I would like to thank Dr. Bruce Dunn for the time that he spent assisting me with my thesis, as well as his advice on my research. Additional thanks are owed to Dr. Karen Hickmann and Dr. Eric Stafne for agreeing to be on my committee and for helping me with abstracts. Thank you to my fellow graduate students: Li Jiang, for your company when I was working on my proposal; Rania, for your help and being someone to talk to about graduate school; and Stephen, for watering my practice grafts and helping me plant at Perkins. Thank you to Leon and Eddy for the use of their land as field plots and a special thanks to Leon for his advice on the sand plums as well as his eagerness to try different graft techniques. -

Apple Scab (Venturia Inaequalis) and Pests in Organic Orchards
Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Boel Sandskär Department of Crop Science, Alnarp Doctoral Thesis Swedish University of Agricultural Sciences Alnarp 2003 2 Abstract Sandskär, B. Apple Scab (Venturia inaequalis) and Pests in Organic Orchards Doctoral Dissertation ISSN 1401-6249, ISBN 91-576-6416-1 Domestication of apples goes back several thousand years in time and archaeological findings of dried apples from Östergötland in Sweden have been dated to ca 2 500 B.C. Worldwide, apples are considered an attractive and healthy fruit to eat. Organic production of apples is increasing abroad but is still at very low levels in Sweden. This study deals with major disease and pest problems in organic growing of apples. It concentrates on the most severe disease, the apple scab (Venturia inaequalis). Resistance to apple scab was evaluated during three years in over 450 old and new apple cultivars at Alnarp and Balsgård in southern Sweden. There were significant differences between the cultivars and years. About ten per cent of the cultivars had a high level of resistance against apple scab. The correlation between foliar and fruit scab was stronger when the scab infection pressure was high (1998-1999), compared to when it was low (2000). Polygenic resistance is a desirable trait since such resistance is more difficult to overcome by the pathogen. A common denominator for polygenic resistance among the cultivars assessed was 'Worcester Pearmain'. The leaf infection of apple scab was compared at three locations: Alnarp, Kivik and Rånna (Skövde) in an observation trial for 22 new apple cultivars. The ranking of the cultivars was similar at the three locations. -

Venturia Paralias Fungal Planet Description Sheets 451
450 Persoonia – Volume 44, 2020 Venturia paralias Fungal Planet description sheets 451 Fungal Planet 1109 – 29 June 2020 Venturia paralias G.C. Hunter, I. Zeil-Rolfe, M. Jourdan & L. Morin, sp. nov. Etymology. Named after Euphorbia paralias, the Euphorbia species from Venturia inaequalis (GenBank MN958659, Identities = 447/468 which the fungus was isolated. (96 %), 1 gap (0 %)). Closest similarities using the tef1-α partial Classification — Venturiaceae, Venturiales, Dothideomy gene sequence were to Venturia polygonivivipari (GenBank cetes. KF853984, Identities 330/358 (92 %), 4 gaps (1 %)), Venturia ditricha (GenBank KF853970, Identities = 327/357 (92 %), Lesions on leaves and stems, amphigenous, predominantly 2 gaps (0 %)) and Venturia chlorospora (GenBank KF 853969, adaxial, circular to irregular, pale to dark brown, 2–8 mm diam, Identities 327/357 (92 %), 2 gaps (0 %)). Venturia paralias was stem lesions pale to dark brown. Mycelium internal, 1.5–6 shown in pathogenicity tests to cause disease on E. paralias mm, subcuticular. Stromata oblong to subcircular, (49-)59– and Euphorbia segetalis (unpubl. data). Venturia paralias is 90(-110) × (29-)39–74(-103) µm, formed by swollen thick- morphologically similar to Fusicladium euphorbiae (Schubert walled cells. Conidiophores in loose to dense fascicles on et al. 2003), which has been recorded from E. amygdaloides, stroma, unbranched, thin-walled, straight to slightly curved, E. cy parissias, E. esula, E. exigua, E. lamprocarpa, E. villosa pale brown and lighter towards the apex, occasionally thickened and E. virgata (Schubert et al. 2003). We were not able to obtain at the base, smooth, (16-)31–59(-81) × (2-)4–5(-6) µm, lectotype material of F. -

Whole Genome Enabled Phylogenetic and Secretome Analyses of Two Venturia Nashicola Isolates
Plant Pathol. J. 36(1) : 98-105 (2020) https://doi.org/10.5423/PPJ.NT.10.2019.0258 The Plant Pathology Journal pISSN 1598-2254 eISSN 2093-9280 ©The Korean Society of Plant Pathology Note Open Access Whole Genome Enabled Phylogenetic and Secretome Analyses of Two Venturia nashicola Isolates Maxim Prokchorchik 1†, Kyungho Won2†, Yoonyoung Lee 1, Cécile Segonzac 3,4, and Kee Hoon Sohn 1,5* 1Department of Life Sciences, Pohang University of Science and Technology, Pohang 37673, Korea 2National Institute of Horticultural and Herbal Science (NIHHS), Rural Development Administration (RDA), Naju 58216, Korea 3Department of Plant Science, Plant Genomics and Breeding Institute and Research Institute of Agriculture and Life Sciences, College of Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea 4Plant Immunity Research Center, College of Agriculture and Life Sciences, Seoul National University, Seoul 08826, Korea 5School of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology, Pohang 37673, Korea (Received on October 11, 2019; Revised on November 29, 2019; Accepted on December 10, 2019) Venturia nashicola is a fungal pathogen causing scab acterization of host determinants in V. nashicola. disease in Asian pears. It is particularly important in the Northeast Asia region where Asian pears are in- Keywords : effector analysis, phylogenetic analysis, Ventu- tensively grown. Venturia nashicola causes disease in ria nashicola Asian pear but not in European pear. Due to the highly restricted host range of Venturia nashicola, it is hypoth- Handling Editor : Sook-Young Park esized that the small secreted proteins deployed by the pathogen are responsible for the host determination. Venturia nashicola is a member of Venturiaceae family Here we report the whole genome based phylogenetic that includes several important fungal pathogens of plant analysis and predicted secretomes for V. -

The Genus Fusicladium (Hyphomycetes) in Poland
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Polish Botanical Society Journals ACTA MYCOLOGICA Dedicated to Professor Alina Skirgiełło Vol. 41 (2): 285-298 on the occasion of her ninety fifth birthday 2006 The genus Fusicladium (Hyphomycetes) in Poland MAŁGORZATA RUSZKIEWICZ MICHALSKA and EWA POŁEĆ 1 Department of Algology and Mycology, University of Łódź, Banacha 12/16, PL 90 237 Łódź [email protected]; [email protected] Ruszkiewicz Michalska M., Połeć E.: The genus Fusicladium (Hyphomycetes) in Poland. Acta Mycol. 41 (2): 285 298, 2006. The paper presents new and historical data on the genus Fusicladium verified on the base of the recently published critical monograph. Fifteen species recorded in Poland under the name Fusicladium and synonymous Pollaccia and Spilocaea are reported; 5 are documented by authors’ materials from Central Poland while the other taxa are supported with literature data only, including three species belonging currently to Fusicladiella and Passalora. Three species, reported here for the first time in Poland: Fusicladium convolvularum Ondřej, F. scribnerianum (Cavara) M. B. Ellis and F. virgaureae Ondřej, are known from a few localities in the world. All the species are provided with the distribution maps and the newly reported ones are illustrated with ink drawings. Key words: parasitic fungi, anamorphic fungi, Deuteromycotina, distribution, Poland INTRODUCTION Worldwide 57 fungal taxa belong to the anamorphic genus Fusicladium Bonord. em. Schubert, Ritschel et U. Braun. They are phytopathologically relevant patho- gens, causing leaf spots, necroses, scab diseases as well as leaf and fruit deformations of members of at least 52 angiospermous plant genera (Schubert, Ritschel, Braun 2003). -

The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae)
The Great Lakes Entomologist Volume 25 Number 3 - Fall 1992 Number 3 - Fall 1992 Article 2 October 1992 The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae) David J. Biddinger Pennsylvania State University Angus J. Howitt Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Biddinger, David J. and Howitt, Angus J. 1992. "The Food Plants and Distribution of the American Plum Borer (Lepidoptera: Pyralidae)," The Great Lakes Entomologist, vol 25 (3) Available at: https://scholar.valpo.edu/tgle/vol25/iss3/2 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Biddinger and Howitt: The Food Plants and Distribution of the American Plum Borer (Lepi 1992 THE GREAT LAKES ENTOMOlOGIST 149 THE FOOD PLANTS AND DISTRIBUTION OF THE AMERICAN PLUM BORER (LEPIDOPTERA: PYRALlDAE)l David J. Biddinger2 and Angus J. Howitt3 ABSTRACT The North American geographical and host plant distributions for the American plnm borer, Euzophera semifuneralis, are reported. Literature and curatorial surveys found the plum borer to be present in 34 states in the U. S. as well as parts of Canada, Mexico, and South America. Pheromone surveys and direct observation found it to be present in high numbers in most cherry and plum orchards in Michigan and in 28 counties of the lower peninsula. A very wide host range representing 15 plant families was found, with most host species in the Rosaceae.