2017 Prunus Crop Germplasm Committee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Production, Pomological and Nutraceutical Properties of Apricot
1 Production, pomological and nutraceutical properties of apricot Khaled Moustafa1* and Joanna Cross2 1Editor of ArabiXiv (arabixiv.org), Paris, France 2Nigde Omer Halisdemir University, Nigde, Turkey Correspondence: [email protected] Abstract Apricot (Prunus sp.) is an important fruit crop worldwide. Despite recent advances in apricot research, much is still to be done to improve its productivity and environmental adaptability. The availability of wild apricot germplasms with economically interesting traits is a strong incentive to increase research panels toward improving its economic, environmental and nutritional characteristics. New technologies and genomic studies have generated a large amount of raw data that the mining and exploitation can help decrypt the biology of apricot and enhance its agronomic values. Here, we outline recent findings in relation to apricot production, pomological and nutraceutical properties. In particular, we retrace its origin from central Asia and the path it took to attain Europe and other production areas around the Mediterranean basin while locating it in the rosaceae family and referring to its genetic diversities and new attempts of classification. The production, nutritional, and nutraceutical importance of apricot are recapped in an easy readable and comparable way. We also highlight and discuss the effects of late frost damages on apricot production over different growth stages, from swollen buds to green fruits formation. Issues related to the length of production season and biotic and abiotic environmental challenges are also discussed with future perspective on how to lengthen the production season without compromising the fruit quality and productivity. Keywords Apricot kernel oil, plum pox virus, prunus armeniaca, spring frost, stone fruit, sharka. -

Nursery Price List
Lincoln-Oakes Nurseries 3310 University Drive • Bismarck, ND 58504 Nursery Seed Price List 701-223-8575 • [email protected] The following seed is in stock or will be collected and available for 2010 or spring 2011 PENDING CROP, all climatic zone 3/4 collections from established plants in North Dakota except where noted. Acer ginnala - 18.00/lb d.w Cornus racemosa - 19.00/lb Amur Maple Gray dogwood Acer tataricum - 15.00/lb d.w Cornus alternifolia - 21.00/lb Tatarian Maple Pagoda dogwood Aesculus glabra (ND, NE) - 3.95/lb Cornus stolonifera (sericea) - 30.00/lb Ohio Buckeye – collected from large well performing Redosier dogwood Trees in upper midwest Amorpha canescens - 90.00/lb Leadplant 7.50/oz Amorpha fruiticosa - 10.50/lb False Indigo – native wetland restoration shrub Aronia melanocarpa ‘McKenzie” - 52.00/lb Black chokeberry - taller form reaching 6-8 ft in height, glossy foliage, heavy fruit production, Corylus cornuta (partial husks) - 16.00/lb NRCS release Beaked hazelnut/Native hazelnut (Inquire) Caragana arborescens - 16.00/lb Cotoneaster integerrimus ‘Centennial’ - 32.00/lb Siberian peashrub European cotoneaster – NRCS release, 6-10’ in height, bright red fruit Celastrus scandens (true) (Inquire) - 58.00/lb American bittersweet, no other contaminating species in area Crataegus crus-galli - 22.00/lb Cockspur hawthorn, seed from inermis Crataegus mollis ‘Homestead’ arnoldiana-24.00/lb Arnold hawthorn – NRCS release Crataegus mollis - 19.50/lb Downy hawthorn Elaeagnus angustifolia - 9.00/lb Russian olive Elaeagnus commutata -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -
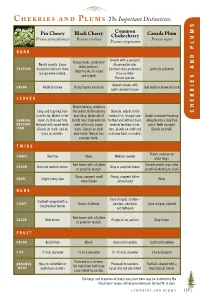
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Pomegranate Concentrate.Indd
Pomegranate Concentrate This delightful fruit is well known in Middle Eastern and Mediterranean cuisines. The red seeds burst with an astringent sweet-tart fl avor. Our Pomegranate Concentrate is made from 100% fresh pomegranates and delivers a powerful, true fruit fl avor. This con- centrate is especially popular is desserts, bar drinks and savory sauces. Product Specifi cs Pomegranate Concentrate Ingredient List: Pomegranate juice concentrate and Serving Size: 1 oz. (28g) fi ltered water Servings per Container: 30 Pack Size: 6/30 oz. wide mouthed HDPE jars per case. Amount Per Serving %Daily Value* Each jar attaches to a standard bar pour spout. Calories 45 Brix: 38 - 40 Total Fat 0g 0% Kosher: Sodium 0mg 0% Conversion: 1- 30 oz. = 0.85 kg Net Wt. Total Carbohydrate 11g 4% 1- 6/30 oz. case = 5.1 kg Net Wt. Dietary Fiber 0g Approx. fl . oz. per jar = 25 fl . oz. Sugars 9g Handling: Keep frozen. Product good for 7-10 days Protein 0g thawed and refrigerated at 40° F and up to 24 months frozen from manufactured date. Not a signifi cant source of calories from fat, saturated fat, trans fat, cholesterol, dietary fi ber, vitamin A, vita- Complimentary Flavors: Walnuts, ginger, orange, min C, calcium and iron. avocado, spicy and bitter lettuce greens *Percent Daily Values are based on a 2,000 calorie diet. Flavor Alternatives: Other high acid, deep colored fl avors like Blood Orange Concentrate, Passion Fruit Concentrate, and Black Currant Interesting... The name pomegranate is derived from the Middle French pome garnete and literally means “apple with many seeds”. -

Prunus X Yedoensis Yoshino Cherry1 Edward F
Fact Sheet ST-523 October 1994 Prunus x yedoensis Yoshino Cherry1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Yoshino Cherry grows quickly to 20 feet, has beautiful bark marked with prominent lenticels but is a relatively short-lived tree (Fig. 1). It has upright to horizontal branching, making it ideal for planting along walks and over patios. The white to pink flowers which occur in early spring before the leaves develop are sometimes damaged by late frosts or very windy conditions. This is the tree along with ‘Kwanzan’ Cherry in Washington, DC, which makes such a show each spring. Figure 1. Mature Yoshino Cherry. GENERAL INFORMATION DESCRIPTION Scientific name: Prunus x yedoensis Pronunciation: PROO-nus x yed-oh-EN-sis Height: 35 to 45 feet Common name(s): Yoshino Cherry Spread: 30 to 40 feet Family: Rosaceae Crown uniformity: symmetrical canopy with a USDA hardiness zones: 5B through 8A (Fig. 2) regular (or smooth) outline, and individuals have more Origin: not native to North America or less identical crown forms Uses: Bonsai; wide tree lawns (>6 feet wide); Crown shape: round; vase shape medium-sized tree lawns (4-6 feet wide); Crown density: moderate recommended for buffer strips around parking lots or Growth rate: medium for median strip plantings in the highway; near a deck Texture: medium or patio; shade tree; narrow tree lawns (3-4 feet wide); specimen; sidewalk cutout (tree pit); no proven urban Foliage tolerance Availability: generally available in many areas within Leaf arrangement: alternate (Fig. 3) its hardiness range Leaf type: simple Leaf margin: double serrate; serrate Leaf shape: elliptic (oval); oblong; ovate Leaf venation: banchidodrome; pinnate Leaf type and persistence: deciduous Leaf blade length: 2 to 4 inches 1. -

Growing Plums, Cherries and Apricots in NH Home Orchards
Bringing information and education into the communities of the Granite State Growing Fruits: Growing Plums, Cherries and Apricots in NH Home Orchards Plums, cherries and apricots, which along with peaches and nec- tarines are often called “stone” fruits, are flavorful additions to the home orchard if the site is suitable. The first consideration is winter hardiness. European plums, hybrid plums, and sour cherries are quite hardy with some varieties tolerating winter temperatures of -20oF or lower. In more protected sites in the Northern part of the state, these stone fruits offer the best chance of success. Japanese plums, apricots and sweet cherries are less hardy and are best suited to home orchards in extreme southern New Hampshire. A second consideration is the risk of spring frost injury to blossoms. These fruits, especially apricots, bloom in very early spring, often a week or more before apple trees bloom. They should be planted on sites that offer freedom from late spring frosts. Generally, these sites are elevated relative to the surrounding landscape which allows cold air to flow away on clear, cold nights. Purchasing Trees Purchase trees from a reputable garden dealer or nursery. There are European plums and sour cherries several mail order nurseries as well that offer quality, bare-root trees. are quite hardy with selected variet- ies hardy to -20o F or more. Japanese Select varieties that are hardy. Most catalogs offer approximate hardi- plums, apricots and sweet cherries are ness ratings. Specific variety recommendations are found below. less hardy. What About Dwarf Trees? All fruit trees are grafted. -

APRICOTS, CANNED Date: July 2012
APRICOTS, CANNED Date: July 2012 PRODUCT DESCRIPTION NUTRITION INFORMATION Apricots are packed in unsweetened fruit juice, light syrup, lightly sweetened fruit ½ cup of apricots count as ½ cup in the juice and water, or lightly sweetened fruit MyPlate.gov Fruit Group. For a 2,000-calorie juice. diet, the daily recommendation is about 2 cups. ½ cup of apricots provides ⅓ of daily of vitamin A needs. PACK/YIELD Each can contains about 15.5 ounces, FOOD SAFETY INFORMATION which is about 1 ½ cups or 3 ½ servings (½ cup each). If the can is leaking or the ends are bulging, throw it away. If the canned food has a bad odor, or liquid STORAGE spurts out when the can is opened, throw it Store unopened cans in a cool, away. clean, dry place. Store remaining opened apricots in a OTHER RESOURCES tightly covered container not made from metal and refrigerate. www.nutrition.gov Look at the “Best if used by” or “Best by” www.choosemyplate.gov date on the can. www.fns.usda.gov/fdd/ For further guidance on how to store and maintain USDA Foods, please visit the NUTRITION FACTS FDD Web site at: http://www.fns.usda.gov/fdd/facts/biubguidance.ht Serving size: 2 canned apricot halves (80g) in m. light syrup Amount Per Serving USES AND TIPS Calories 50 Calories from Fat 0 Canned apricots are a delicious dessert or snack served directly from the can. They % Daily Value* can be served chilled or at room Total Fat 0 g 0% temperature. Saturated Fat 0 g 0% Freeze the drained juice in an ice cube tray and use instead of ice cubes to sweeten Trans Fat 0g cold drinks like iced tea. -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

Plums, Nectarines, Apricots, Cherries, Almonds and Prunus Hybrids
E-612 2-13 Texas Fruit and Nut Production lums, Nectarines, Apricots Cherries, Almonds and Prunus hybrids Larry Stein, Jim Kamas, and Monte Nesbitt Extension Fruit Specialists, The Texas A&M University System s closely related members of the rose family, plums and apricots typically require similar management. Both fruits have performed Amuch better in Texas than nectarines, almonds, sweet cherries, and Prunus hybrids because they are less susceptible to disease, varmints, and crop loss due to premature blooming. Plums The plum tree has white flowers and sets fruit on buds from previous season’s growth (Fig. 1). Usually Figure 1. A plum orchard in full bloom. the fruit has a dusty white coating or wax bloom that is easily rubbed off (Fig. 2). Plums can be sweet to tart; the skin is typically quite tart. The two main species used in the United States are the European plum, Prunus domestica, and the Japanese plum, Prunus salycina. The European plum includes varieties such as ‘Stanley’, which is grown for fresh fruit and often dried for use as prunes. These varieties have produced poorly in Texas because they require cold climates and are susceptible to fungal diseases such as brown rot. The varieties adapted to Texas are usually hybrids Figure 2. The dusty white coating associated with between P. domestica and P. salycina and are known plums is known as wax bloom. 1 Figure 4. Eating a ripe, juicy Figure 5. ‘Bruce’ plums. ‘Methley’ plum right off the tree. as Japanese or Japanese hybrid varieties. Most plum varieties are not self-fruitful.