The Hand of Homonaledi
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
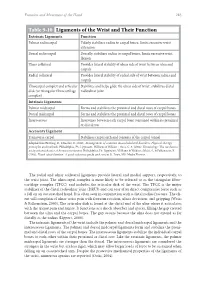
Table 9-10 Ligaments of the Wrist and Their Function
Function and Movement of the Hand 283 Table 9-10 Ligaments of the Wrist and Their Function Extrinsic Ligaments Function Palmar radiocarpal Volarly stabilizes radius to carpal bones; limits excessive wrist extension Dorsal radiocarpal Dorsally stabilizes radius to carpal bones; limits excessive wrist flexion Ulnar collateral Provides lateral stability of ulnar side of wrist between ulna and carpals Radial collateral Provides lateral stability of radial side of wrist between radius and carpals Ulnocarpal complex and articular Stabilizes and helps glide the ulnar side of wrist; stabilizes distal disk (or triangular fibrocartilage radioulnar joint complex) Intrinsic Ligaments Palmar midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Dorsal midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Interosseous Intervenes between each carpal bone contained within its proximal or distal row Accessory Ligament Transverse carpal Stabilizes carpal arch and contents of the carpal tunnel Adapted from Hertling, D., & Kessler, R. (2006). Management of common musculoskeletal disorders: Physical therapy principles and methods. Philadelphia, PA: Lippincott, Williams & Wilkins.; Oatis, C. A. (2004). Kinesiology: The mechanics and pathomechanics of human movement. Philadelphia, PA: Lippincott, Williams & Wilkins.; Weiss, S., & Falkenstein, N. (2005). Hand rehabilitation: A quick reference guide and review. St. Louis, MO: Mosby Elsevier. The radial and ulnar collateral ligaments provide lateral and medial support, respectively, to the wrist joint. The ulnocarpal complex is more likely to be referred to as the triangular fibro- cartilage complex (TFCC) and includes the articular disk of the wrist. The TFCC is the major stabilizer of the distal radioulnar joint (DRUJ) and can tear after direct compressive force such as a fall on an outstretched hand. -

Carpals and Tarsals of Mule Deer, Black Bear and Human: an Osteology Guide for the Archaeologist
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2009 Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist Tamela S. Smart Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Anthropology Commons Recommended Citation Smart, Tamela S., "Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist" (2009). WWU Graduate School Collection. 19. https://cedar.wwu.edu/wwuet/19 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. MASTER'S THESIS In presenting this thesis in partial fulfillment of the requirements for a master's degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWu. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. I acknowledge that I retain ownership rights to the copyright of this work, including but not limited to the right to use all or part of this work in future works, such as articles or books. -

Bone Limb Upper
Shoulder Pectoral girdle (shoulder girdle) Scapula Acromioclavicular joint proximal end of Humerus Clavicle Sternoclavicular joint Bone: Upper limb - 1 Scapula Coracoid proc. 3 angles Superior Inferior Lateral 3 borders Lateral angle Medial Lateral Superior 2 surfaces 3 processes Posterior view: Acromion Right Scapula Spine Coracoid Bone: Upper limb - 2 Scapula 2 surfaces: Costal (Anterior), Posterior Posterior view: Costal (Anterior) view: Right Scapula Right Scapula Bone: Upper limb - 3 Scapula Glenoid cavity: Glenohumeral joint Lateral view: Infraglenoid tubercle Right Scapula Supraglenoid tubercle posterior anterior Bone: Upper limb - 4 Scapula Supraglenoid tubercle: long head of biceps Anterior view: brachii Right Scapula Bone: Upper limb - 5 Scapula Infraglenoid tubercle: long head of triceps brachii Anterior view: Right Scapula (with biceps brachii removed) Bone: Upper limb - 6 Posterior surface of Scapula, Right Acromion; Spine; Spinoglenoid notch Suprspinatous fossa, Infraspinatous fossa Bone: Upper limb - 7 Costal (Anterior) surface of Scapula, Right Subscapular fossa: Shallow concave surface for subscapularis Bone: Upper limb - 8 Superior border Coracoid process Suprascapular notch Suprascapular nerve Posterior view: Right Scapula Bone: Upper limb - 9 Acromial Clavicle end Sternal end S-shaped Acromial end: smaller, oval facet Sternal end: larger,quadrangular facet, with manubrium, 1st rib Conoid tubercle Trapezoid line Right Clavicle Bone: Upper limb - 10 Clavicle Conoid tubercle: inferior -
Hand Bone Age: a Digital Atlas of Skeletal Maturity
V. Gilsanz/O. Ratib · Hand Bone Age Vicente Gilsanz · Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity With 88 Figures Vicente Gilsanz, M.D., Ph.D. Department of Radiology Childrens Hospital Los Angeles 4650 Sunset Blvd., MS#81 Los Angeles, CA 90027 Osman Ratib, M.D., Ph.D. Department of Radiology David Geffen School of Medicine at UCLA 100 Medical Plaza Los Angeles, CA 90095 This eBook does not include ancillary media that was packaged with the printed version of the book. ISBN 3-540-20951-4 Springer-Verlag Berlin Heidelberg New York Library of Congress Control Number: 2004114078 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable to prosecution under the German Copyright Law. Springer-Verlag Berlin Heidelberg New York Springer is a part of Springer Science+Business Media http://www.springeronline.com A Springer-Verlag Berlin Heidelberg 2005 Printed in Germany The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from therelevantprotectivelawsandregulationsandthereforefreeforgeneraluse. Product liability: The publishers cannot guarantee the accuracy of any information about the application of operative techniques and medications contained in this book. -

Bones of Upper Limb
BONES OF THE UPPER LIMB Dr. Jamila El-Medany OBJECTIVES At the end of the lecture, students should be able to: List the different bones of the UL. List the characteristic features of each bone. Differentiate between the bones of the right and left sides. List the articulations between the different bones. The Bones of UL are: Pectoral Girdle. Arm : Humerus. Forearm : Radius & Ulna. Wrist : Carpal bones Hand: Metacarpals & Phalanges Pectoral Girdle Formed of Two Bones: Clavicle (anteriorly) and Scapula (posteriorly). It is very light and allows the upper limb to have exceptionally free movement. Clavicle It is a doubly curved long bone lying horizontally across the root of the neck It is subcutaneous throughout its length. Functions: 1. It serves as a rigid support from which the scapula and free upper limb are suspended & keep them away from the trunk so that the arm has maximum freedom of movement. 2. Transmits forces from the upper limb to the axial skeleton. 3. Provides attachment for muscles. 4. It forms a boundary of the Cervicoaxillary canal for protection of the neurovascular bundle of the UL. Clavicle It is a long bone with no medullary cavity. It has the appearance of an elongated letter Capital (S) lying on one side. It has Two Ends: Medial (Sternal) : enlarged & triangular. Lateral (Acromial) : flattened. Body (shaft): Its medial 2/3 is convex forward. Its lateral 1/3 is concave forward. Surfaces: Superior : smooth as it lies just deep to the skin. Inferior : rough because strong ligaments bind it to the 1st rib. Articulations of Clavicle Medially with the manubrium at the Sternoclavicular joint . -

Macroanatomy of the Bones of Thoracic Limb of an Asian Elephant (Elephas Maximus)
Int. J. Morphol., 34(3):909-917, 2016. Macroanatomy of the Bones of Thoracic Limb of an Asian Elephant (Elephas maximus) Macroanatomía de los Huesos del Miembro Torácico de un Elefante Asiático (Elephas maximus) A. S. M. Lutful Ahasan*; Md. Abul Quasem*; Mohammad Lutfur Rahman*; Rubyath Binte Hasan*; A. S. M. Golam Kibria* & Subrata Kumar Shil* AHASAN, A. M. S. L.; QUASEM, M. A.; RAHMAN, M. L.; HASAN, R. B.; KIBRIA, A. S. M. G. & SHIL, S. K. Macroanatomy of the bones of thoracic limb of an Asian Elephant (Elephas maximus). Int. J. Morphol., 34(3):909-917, 2016. SUMMARY: Bones of forelimb were studied from a prepared skeleton of an adult female Asian elephant (Elephas maximus) in Anatomy Museum of Chittagong Veterinary and Animal Sciences University to understand the morphological form and structure of Asian elephant forelimb. The angle was approximately 123º between caudal border of scapula and caudal border of humerus. The scapula, humerus and bones of the antebrachium (particularly the ulna) were massive bones. The bones of manus were the short and relatively small. The dorsal border of scapula extended from the level of proximal extremity of first rib to the middle of the 6th rib. Ventral angle of scapula articulated with humerus by elongated shaped glenoid cavity (cavitas glenoidalis) of scapula and head of humerus (caput humeri). The major tubercle (tuberculum majus) of humerus was situated laterally to the head, which had smaller cranial part with large caudal part and extended cranially to the head. The crest of minor tubercle (tuberculum minus) was present as the rough line on the mediocaudal surface of humerus that ends in a slight depressed or elevated area, known as teres major tuberosity (tuberositas teres major). -

Upper Limb 3 the Wrist
Upper Limb 3 The Wrist Donald Sammut Hand Surgeon Kings Upper Limb Anatomy plus lecture notes • Unlike'many'other'joints,'the'wrist'surface'anatomy'gives'away'little'of' the'bony'structures'beneath'the'surface.' • Still'less'does'it'suggest'the'complex'ligament'structures' Carpus' Radius' Ulna' • The'wrist'is'surrounded'by'vital'structures,'tendons,'nerves,'arteries.'' • Pathology'in'these'structures'can'give'symptoms'mistaken'for'problems' with'the'wrist'joint' • 8'wrist'bones'form'the'carpus.' • Proximally'these'articulate'with'the'radius' • Distally'they'articulate'with'the'metacarpals.' • The'carpal'bones'articulate'with'each'other'in'a'particular'configuration'of' bony'shapes'and'ligaments'which'dictate'the'complex'function.' Trapezoid' Capitate' Trapezium' Hamate' Scaphoid' Pisiform' Lunate' Triquetral' • Proximally'the'carpus'articulates'with'the'distal'radius'and'with'the' triangular'fibrocartilage.' • The'TFC'separates'the'carpus'from'the'ulna' • The'distal'ulna'does'not'participate'in'the'articulation'with'the'carpus' • View'of'the'distal'radius.'Proximal'aspect'of'the'RadioKcarpal'joint' • Note'' 'the'Scaphoid'fossa' 'the'Lunate'fossa' 'the'triangular'fibrocartilage' PROXIMAL)ARTICULAR)SURFACE)OF)THE)WRIST:)RADIUS'AND'TRIANGULAR'CARTILAGE' ' THE'ULNA'IS'EXCLUDED.' • Distal'aspect'of'the'RadioKcarpal'joint' The'radius'and'triangular'fibrocartilage'articulate'with'the'scaphoid,'lunate' and'triquetral' DISTAL)ARTICULAR)SURFACE)OF)THE)WRIST:)SCAPHOID,'LUNATE','TRIQUETRAL' ' • The'bony'shapes'of'the'radiocarpal'joint'make'for'an'unstable'arrangement' -

Redalyc.Age Determination in Young Keeshound Puppies Using a Simple
Multiciencias ISSN: 1317-2255 [email protected] Universidad del Zulia Venezuela Alvarado Morillo, Manuel Age Determination in young Keeshound Puppies Using a simple Radiographic Study of the Radius, Ulna and Carpus Multiciencias, vol. 7, núm. 3, septiembre-diciembre, 2007, pp. 249-255 Universidad del Zulia Punto Fijo, Venezuela Available in: http://www.redalyc.org/articulo.oa?id=90470302 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciencias del Agro y del Mar MULTICIENCIAS, Vol. 7, Nº 3, 2007 (249 - 255) ISSN 1317-2255 / Dep. legal pp. 200002FA828 Age Determination in young Keeshound Puppies Using a simple Radiographic Study of the Radius, Ulna and Carpus Manuel Alvarado Morillo Cátedra de Radiología Clínica e Imagenología. Departamento Médico Quirúrgico. Facultad de Ciencias Veterinarias. Universidad del Zulia P.O.Box 15157 (C.C. Galerias Mall) Maracaibo, Venezuela. E-mail: [email protected] Abstract Radiographic features in the normal development of the radius, ulna and carpal bones from birth to maturity in medium size dogs are reported in order to have an important tool for determining a dog’s age from radiographs. Eight (male and female) Keeshounds from the ages of one day to eleven months were studied to determine the normal radiographic development of the radius, ulna and carpal bones. At birth, diaphyses of the radius and ulna are present. The epiphyses and carpal bones are absent radiographically because they are composed of cartilaginous tissue. -

Anatomical Characteristic of Forelimb Skeleton of Sumatran Rhino (Dicerorhinus Sumatrensis)
Proceeding of the 5th Congress of Asian Association of Veterinary Anatomists Bali- INDONESIA, February 12-13th, 2015 AH-03 Anatomical Characteristic of Forelimb Skeleton of Sumatran Rhino (Dicerorhinus sumatrensis) Nurhidayat*, Eni Puji Lestari, Chairun Nisa’, Danang Dwi Cahyadi, Supratikno Department of Anatomy Physiology and Pharmacology, Faculty of Veterinary Medicine, Bogor Agricultural University, Bogor 16680, Indonesia *Corresponding author: [email protected] Keywords: Sumatran rhino, anatomy, forelimb skeleton INTRODUCTION Sumatran rhino is one of the endangered animal species of Indonesia. This animal belongs to the order Perissodactyla, family Rhinocerotidae and genus Dicerorhinus. Although the animal’s weight reaches 1.000 kgs, Sumatran rhino is the smallest among family Rhinocerotidae (Van Strien 1974). To support the big size and rounded body, Sumatran rhino has relatively short legs with three toes on each. The body’s structure is adjusted with the animal’s behavior to move quickly, even to be able to climb the sheer cliffs. In general, the forelimb get bigger burden than that the hindlimb to perform the daily activities, support the weight of the body, neck and head, so that the field wider footprint pivot (De Blasé dan Martin 1981). Therefore, Sumatran rhino’s forelimb skeleton need to be studied to provide information about the relationship between the characteristics of the skeletal structure of the forelimb related to their function. MATERIALS AND METHODS The study was used a set of forelimb skeleton of female Sumatran rhino (named: Dusun) aged around 20 years old that received from Sumatran Rhino Sanctuary (SRS), Way Kambas National Park, Lampung, Indonesia. This study was done by observing the Sumatran rhino’s forelimb skeleton in detail. -

Radiographic Evaluation of the Wrist: a Vanishing Art Rebecca A
Radiographic Evaluation of the Wrist: A Vanishing Art Rebecca A. Loredo, MD,* David G. Sorge, MD, Lt. Colonel,† and Glenn Garcia, MD‡ he intricate anatomy and compartmentalization of struc- interpretation of standard or MR arthrograms and for identi- Ttures in the wrist are somewhat daunting. As in other joints, fying various patterns of arthritic involvement.2 The com- the radiographic appearance of disease processes affecting the partments are as follows: wrist is very much dependent on the articular and periarticular soft tissue and osseous anatomy. Therefore, abbreviated discus- 1. Radiocarpal compartment sions of the pertinent anatomy are included within the introduc- 2. Midcarpal compartment tion with more specific anatomic discussions within the text as a 3. Pisiform-triquetral compartment prelude to certain conditions affecting the wrist. 4. Common carpometacarpal compartment 5. First carpometacarpal compartment 6. Intermetacarpal compartments Anatomy of the Wrist 7. Inferior (distal) radioulnar compartment Osseous Anatomy In daily clinical practice, the most important compart- The osseous structures of the wrist are the distal portions of the ments are the radiocarpal, midcarpal, and distal radioulnar radius and ulna, the proximal and distal rows of carpal bones, compartments. The radiocarpal compartment (Fig. 2) lies and the bases of the metacarpals (Fig. 1). The proximal row of between the proximal carpal row and the distal radius and carpal bones consists of the scaphoid, lunate, triquetrum, and the triangular fibrocartilage, which is fibrocartilaginous tis- the pisiform. The distal row of carpal bones contains the trape- sue that extends from the ulnar side of the distal aspect of the zium, trapezoid, capitate, and hamate bones. -
Bones and Joints of the Upper Limb. Sándor Katz M.D., Ph.D
Bones and joints of the upper limb. Sándor Katz M.D., Ph.D. Anatomy It is a branch of biology concerned with the study of the structure organisms and their parts. Human anatomy is one of the basic essential sciences of medicine. ana=up tom, tomia= cut, dissect cut up or cut open Anatomy The discipline of anatomy is divided into macroscopic and microscopic anatomy. Macroscopic anatomy or gross anatomy is the examination of body parts with naked eye. Microscopic anatomy involves the use of optical instruments in the study of the tissues of various structures, known as histology. Anatomy Macroscopic anatomy describes the … • location • shape • external surface • internal surface • parts of organs or structures. Course books Course books Anatomical planes (overview) Skeleton Function of bones • support • movements - muscle attachment • protection • Ca storage • bone marrow - hemopoiesis Identifying characteristics • fossa - depression • sinus - cavity • foramen - hole • meatus - tube • condyles - large, smooth and curved surfaces • tubercle - projection • tuberosity - rough surface Classification of bones • long bones • short bones • irregular bones • flat bones • pneumatic bones • sesamoid bones Long bones - general features e.g. humerus, femur proximal epiphysis Long bones are mostly presented in the diaphysis=shaft=body appendicular skeleton, and they facilitate movements. distal epiphysis Short bones - general features e.g. carpal and tarsal bones Short bones provide stability and some movements. Irregular bones - general features e.g. vertebrae Irregular bones have a complex shape, which helps to protect organs. Flat bones - general features e.g. scapula, sternum and ribs Flat bones can provide protection (like a shield) and large areas of attachment for muscles. -

Geometric and Mechanical Characterization of Human Carpal Bones – a Preliminary Study
https://doi.org/10.3311/PPci.15125 138|Creative Commons Attribution b Periodica Polytechnica Civil Engineering, 64(1), pp. 138–143, 2020 Geometric and Mechanical Characterization of Human Carpal Bones – a Preliminary Study Dénes Faragó1,2, Rita M. Kiss1,2* 1 Cooperation Research Center for Biomechanics, Faculty of Mechanical Engineering, Budapest University of Technology and Economics, Budapest, Hungary , 1111 Budapest, Műegyetem rkp. 3. 2 Department of Mechatronics, Optics and Mechanical Engineering Informatics, Faculty of Mechanical Engineering, Budapest University of Technology and Economics, Budapest, Hungary, 1111 Budapest, Műegyetem rkp. 3 * Corresponding author, e-mail: [email protected] Received: 15 October 2019, Accepted: 21 October 2019, Published online: 15 January 2020 Abstract Human hand injuries account for a significant number of accidents of young adults (mostly sports injuries) and elderly people. The most vulnerable part of the hand is the wrist, a construct consisting of numerous bones and ligaments. The hand is a complex structure, the mechanical behavior is hard to describe, and also it is sometimes hard to correctly diagnose the injuries. The goal of the present research is to create a quickly and inexpensive measurement method to characterize the geometrical and mechanical properties of carpal bones. The method presented is suitable to properly characterize the intact and damaged geometries of different carpal bones (capitate scaphoid, trapezium, pisiform). 3D models of intact and failed bones are determined by a 3D scanner, mechanical properties are determined with high-speed compression load (700 mm/min), which represents the fracture by falling down. According to the test results, the 3D scanning technique provided valuable geometrical data for cross-section calculation (scan before the test) and for analysis of the failure mode of the bones (scan after the test).