Hand Bone Age: a Digital Atlas of Skeletal Maturity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association Between Dietary Habits and Parental Health with Obesity Among Children with Precocious Puberty
children Article Association between Dietary Habits and Parental Health with Obesity among Children with Precocious Puberty 1, 1, 2 1 3, Yong Hee Hong y , Yeon Ju Woo y, Jong Hyun Lee , Young-Lim Shin and Hee-Sook Lim * 1 Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University School of Medicine, Bucheon 14584, Korea; [email protected] (Y.H.H.); [email protected] (Y.J.W.); [email protected] (Y.-L.S.) 2 Department of Pediatrics, Soonchunhyang University Gumi Hospital, Soonchunhyang University School of Medicine, Gumi 39371, Korea; [email protected] 3 Department of Food Sciences and Nutrition, Yeonsung University, Anyang 14011, Korea * Correspondence: [email protected] These authors contributed equally to this work as first author. y Received: 25 September 2020; Accepted: 5 November 2020; Published: 8 November 2020 Abstract: Precocious puberty, resulting in various physical, mental, and social changes, may have negative consequences for children and their families. In this study, we investigated whether there were differences between parental obesity, children’s and parent’s awareness of body shape, and dietary habits according to obesity levels in children with precocious puberty. A total of 193 children (93.3% girls) diagnosed with precocious puberty were classified into three groups according to their obesity levels. Negative body shape awareness and dissatisfaction were significantly higher in the obese group than in the normal-weight group, and parents were more likely to perceive their children as fat than the children themselves. In addition, the obesity rate of parents in the obese group was higher, and the body mass indexes of children and parents were significantly correlated. -

Applications of Chondrocyte-Based Cartilage Engineering: an Overview
Hindawi Publishing Corporation BioMed Research International Volume 2016, Article ID 1879837, 17 pages http://dx.doi.org/10.1155/2016/1879837 Review Article Applications of Chondrocyte-Based Cartilage Engineering: An Overview Abdul-Rehman Phull,1 Seong-Hui Eo,1 Qamar Abbas,1 Madiha Ahmed,2 and Song Ja Kim1 1 Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongjudaehakro 56, Gongju 32588, Republic of Korea 2Department of Pharmacy, Quaid-i-Azam University, Islamabad 45320, Pakistan Correspondence should be addressed to Song Ja Kim; [email protected] Received 14 May 2016; Revised 24 June 2016; Accepted 26 June 2016 Academic Editor: Magali Cucchiarini Copyright © 2016 Abdul-Rehman Phull et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chondrocytes are the exclusive cells residing in cartilage and maintain the functionality of cartilage tissue. Series of biocomponents such as different growth factors, cytokines, and transcriptional factors regulate the mesenchymal stem cells (MSCs) differentiation to chondrocytes. The number of chondrocytes and dedifferentiation are the key limitations in subsequent clinical application of the chondrocytes. Different culture methods are being developed to overcome such issues. Using tissue engineering and cell based approaches, chondrocytes offer prominent therapeutic option specifically in orthopedics for cartilage repair and to treat ailments such as tracheal defects, facial reconstruction, and urinary incontinence. Matrix-assisted autologous chondrocyte transplantation/implantation is an improved version of traditional autologous chondrocyte transplantation (ACT) method. An increasing number of studies show the clinical significance of this technique for the chondral lesions treatment. -

Copyrighted Material
C01 10/31/2017 11:23:53 Page 1 1 1 The Normal Anatomy of the Neck David Bainbridge Introduction component’ of the neck is a common site of pathology, and the diverse forms of neck The neck is a common derived characteristic disease reflect the sometimes complex and of land vertebrates, not shared by their aquatic conflicting regional variations and functional ancestors. In fish, the thoracic fin girdle, the constraints so evident in this region [2]. precursor of the scapula, coracoid and clavi- Unlike the abdomen and thorax, there is no cle, is frequently fused to the caudal aspect of coelomic cavity in the neck, yet its ventral part the skull. In contrast, as vertebrates emerged is taken up by a relatively small ‘visceral on to the dry land, the forelimb separated from compartment’, containing the larynx, trachea, the head and the intervening vertebrae speci- oesophagus and many important vessels, alised to form a relatively mobile region – the nerves and endocrine glands. However, I neck – to allow the head to be freely steered in will not review these structures, as they do many directions. not represent an extension of the equine ‘back’ With the exception of the tail, the neck in the same way that the more dorsal locomo- remains the most mobile region of the spinal tor region does. column in modern-day horses. It permits a wide range of sagittal plane flexion and exten- sion to allow alternating periods of grazing Cervical Vertebrae 3–7 and predator surveillance, as well as frontal plane flexion to allow the horizon to be scan- Almost all mammals, including the horse, ned, and rotational movement to allow possess seven cervical vertebrae, C1 to C7 nuisance insects to be flicked off. -

Meyer Dysplasia in the Differential Diagnosis of Hip Disease in Young Children
ARTICLE Meyer Dysplasia in the Differential Diagnosis of Hip Disease in Young Children Liora Harel, MD; Liora Kornreich, MD; Shai Ashkenazi, MD, MSc; Avinoam Rachmel, MD; Boaz Karmazyn, MD; Jacob Amir, MD Objectives: To describe a rare developmental disorder Results: Two of the 5 patients were initially diagnosed of the femoral capital epiphysis in infants and children as having osteomyelitis and 3 as having Perthes disease. that is often misdiagnosed and to suggest an evaluation The diagnosis of Meyer dysplasia was confirmed by plain protocol to differentiate it from other hip problems. film of the pelvis, a negative bone scan, or normal bone marrow findings on magnetic resonance imaging. The Design: Case series. limping resolved without treatment in all patients within 1 to 3 weeks. Setting: Tertiary care center. Conclusions: Meyer dysplasia is a benign condition that Subjects: Five consecutive patients referred for evalu- should be included in the differential diagnosis of hip dis- ation of acute onset of limping between January 1990 and ease in infants and children. Awareness of this condi- December 1997. tion may prevent unnecessary hospitalization and treat- ment. Intervention: All clinical and imaging data were col- lected. Arch Pediatr Adolesc Med. 1999;153:942-945 5 patients. The 5 children included 4 males Editor’s Note: Keep this in mind next time you see a limping and 1 female aged 9 to 48 months. All pre- toddler or preschooler. sented with acute onset of limping of 2 to Catherine D. DeAngelis, MD 30 days’ duration. Patient 3 was being ob- served by a pediatric endocrinologist for short stature, and patient 4 had a family EYER dysplasia is a history of congenital dislocation of the hip symptomless develop- and Perthes disease. -

Identification of Teeth, Wrist and Femur Bone Features for Age and Gender Identifiction
Vidyashree H S et al. / International Journal of Computer Science Engineering (IJCSE) IDENTIFICATION OF TEETH, WRIST AND FEMUR BONE FEATURES FOR AGE AND GENDER IDENTIFICTION Vidyashree H S Department of Computer Science and Engineering, Bapuji Institute of Engineering and Technology, Davanagere, India. Email: [email protected] Pradeep N Department of Computer Science and Engineering, Bapuji Institute of Engineering and Technology, Davanagere, India. Email: [email protected] Abstract: Personal identification is defined as establishing the identity of an individual. The need for personal identification arises in natural mass disasters like earth quakes, tsunamis, landslides, floods etc., and in man-made disasters such as terrorist attacks, bomb blasts, mass murders, and in cases when the body is highly decomposed or dismembered to deliberately conceal the identity of the individual. Computers have been widely used in the field of medical research over the past few decades. Machine Learning and Biomedical Image Processing Techniques have enormously contributed in the field of Medical Image Analysis, classification and recognition. But fewer contributions have been made in the area of forensics by researchers. There is a scope for researches, where they can make their contributions especially in medical image analysis where they can estimate age and identify gender using digital X-ray images. In this paper, we are identifying features of Femur, Teeth and Wrist features that are helpful for age and gender identification. Keywords- Forensic Science, Femur Bone Age, Gender Estimation, teeth, wrist bone age and gender comparison. I.INTRODUCTION The age of an individual is often a fundamental piece of data in connection with forensic identification of unidentified bodies. -

Development of the Endochondral Skeleton
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Development of the Endochondral Skeleton Fanxin Long1,2 and David M. Ornitz2 1Department of Medicine, Washington University School of Medicine, St. Louis, Missouri 63110 2Department of Developmental Biology, Washington University School of Medicine, St. Louis, Missouri 63110 Correspondence: fl[email protected] SUMMARY Much of the mammalian skeleton is composed of bones that originate from cartilage templates through endochondral ossification. Elucidating the mechanisms that control endochondral bone development is critical for understanding human skeletal diseases, injury response, and aging. Mouse genetic studies in the past 15 years have provided unprecedented insights about molecules regulating chondrocyte formation, chondrocyte maturation, and osteoblast differ- entiation, all key processes of endochondral bone development. These include the roles of the secreted proteins IHH, PTHrP, BMPs, WNTs, and FGFs, their receptors, and transcription factors such as SOX9, RUNX2, and OSX, in regulating chondrocyte and osteoblast biology. This review aims to integrate the known functions of extracellular signals and transcription factors that regulate development of the endochondral skeleton. Outline 1 Introduction 5 Osteoblastogenesis 2 Mesenchymal condensation 6 Closing remarks 3 Chondrocyte differentiation References 4 Growth plate development Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant Additional Perspectives on Mammalian Development available at www.cshperspectives.org Copyright # 2013 Cold Spring Harbor Laboratory Press; all rights reserved; doi: 10.1101/cshperspect.a008334 Cite this article as Cold Spring Harb Perspect Biol 2013;5:a008334 1 Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press F. -

Female Tanner Stages (Sexual Maturity Rating)
Strength of Recommendations Preventive Care Visits – 6 to 17 years Bold = Good Greig Health Record Update 2016 Italics = Fair Plain Text = consensus or Selected Guidelines and Resources – Page 3 inconclusive evidence The CRAFFT Screening Interview Begin: “I’m going to ask you a few questions that I ask all my patients. Please be honest. I will keep your Screening for Major Depressive Disorder -USPSTF answers confidential.” Age 12 years to 18 years 7 to 11 yrs No Yes Part A During the past 12 months did you: Screen (when systems in place for diagnosis, treatment and Insufficient 1. Drink any alcohol (more than a few sips)? □ □ follow-up) evidence 2. Smoked any marijuana or hashish? □ □ Risk factors- parental depression, co-morbid mental health or chronic medical 3. Used anything else to get high? (“anything else” includes illegal conditions, having experienced a major negative life event drugs, over the counter and prescription drugs and things that you sniff or “huff”) □ □ Tools-Patient Health Questionnaire for Adolescent(PHQ9-A) Tools For clinic use only: Did the patient answer “yes” to any questions in Part A? &Beck Depression Inventory-Primary Care version (BDI-PC) perform less No □ Yes □ well Ask CAR question only, then stop. Ask all 6 CRAFFT questions Treatment-Pharmacotherapy – fluoxetine (a SSRI) is Part B Have you ever ridden in a CAR driven by someone □ □ efficacious but SSRIs have a risk of suicidality – consider only (including yourself) who was ‘‘high’’ or had been using if clinical monitoring is possible. Psychotherapy alone or alcohol or drugs? combined with pharmacotherapy can be efficacious. -

Studies on Brain and Spinal Cord Tumors
Studies on Brain and Spinal Cord Tumors Chapter 1 Osteochondroma of the Spine Iraj Lotfinia Professor of Neurosurgery, Tabriz Universsity of medical science, Tabriz, Iran. Fax: 00984113340830; Email: [email protected] Abstract Osteochondroma (OC) is the most common benign tumor of the bones, and it remains the most common precursor for secondary chondrosarcoma, which often occurs in the long bones’ metaphyseal areas. Rarely, it is also found in the spine. This tumor comprises a cartilage capped bone projection and is observed in both solitary and multiple forms. In many cases, the lesion can be definitively diagnosed according to radiological characteristics, but the rarity of these lesions in the spine, gradual onset of symptoms, and the frequent lack of observation of lesions in plain radiography may delay the diagnosis or cause misdiagnosis. These lesions are be- nign and do not risk the patient’s life; however, they rarely may be found to be a malignant degeneration that transformed into chondrosarcoma. When the lesion has led to clinical symptoms or has faced the patient with cosmetic challenges, or when definitive diagnosis is unknown, treatment is required. The primary treatment is the surgical removal of the lesion. Timely diagnosis and complete resection of the le- sion using surgery lead to complete recovery and prevent recurrence. 1. Introduction According to the World Health Organization’s (WHO’s) definition in 2002, osteocar- tilaginous exostosis are benign bone neoplasms covered by a cartilaginous cap created at the outer surface of the bone by endochondral ossification [1]. Osteochondroma (OC) is the most common benign primary tumor of the bone. -
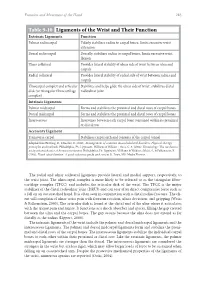
Table 9-10 Ligaments of the Wrist and Their Function
Function and Movement of the Hand 283 Table 9-10 Ligaments of the Wrist and Their Function Extrinsic Ligaments Function Palmar radiocarpal Volarly stabilizes radius to carpal bones; limits excessive wrist extension Dorsal radiocarpal Dorsally stabilizes radius to carpal bones; limits excessive wrist flexion Ulnar collateral Provides lateral stability of ulnar side of wrist between ulna and carpals Radial collateral Provides lateral stability of radial side of wrist between radius and carpals Ulnocarpal complex and articular Stabilizes and helps glide the ulnar side of wrist; stabilizes distal disk (or triangular fibrocartilage radioulnar joint complex) Intrinsic Ligaments Palmar midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Dorsal midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Interosseous Intervenes between each carpal bone contained within its proximal or distal row Accessory Ligament Transverse carpal Stabilizes carpal arch and contents of the carpal tunnel Adapted from Hertling, D., & Kessler, R. (2006). Management of common musculoskeletal disorders: Physical therapy principles and methods. Philadelphia, PA: Lippincott, Williams & Wilkins.; Oatis, C. A. (2004). Kinesiology: The mechanics and pathomechanics of human movement. Philadelphia, PA: Lippincott, Williams & Wilkins.; Weiss, S., & Falkenstein, N. (2005). Hand rehabilitation: A quick reference guide and review. St. Louis, MO: Mosby Elsevier. The radial and ulnar collateral ligaments provide lateral and medial support, respectively, to the wrist joint. The ulnocarpal complex is more likely to be referred to as the triangular fibro- cartilage complex (TFCC) and includes the articular disk of the wrist. The TFCC is the major stabilizer of the distal radioulnar joint (DRUJ) and can tear after direct compressive force such as a fall on an outstretched hand. -

Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals
J Mammal Evol DOI 10.1007/s10914-014-9256-7 ORIGINAL PAPER Intervertebral and Epiphyseal Fusion in the Postnatal Ontogeny of Cetaceans and Terrestrial Mammals Meghan M. Moran & Sunil Bajpai & J. Craig George & Robert Suydam & Sharon Usip & J. G. M. Thewissen # Springer Science+Business Media New York 2014 Abstract In this paper we studied three related aspects of the Introduction ontogeny of the vertebral centrum of cetaceans and terrestrial mammals in an evolutionary context. We determined patterns The vertebral column provides support for the body and of ontogenetic fusion of the vertebral epiphyses in bowhead allows for flexibility and mobility (Gegenbaur and Bell whale (Balaena mysticetus) and beluga whale 1878;Hristovaetal.2011; Bruggeman et al. 2012). To (Delphinapterus leucas), comparing those to terrestrial mam- achieve this mobility, individual vertebrae articulate with each mals and Eocene cetaceans. We found that epiphyseal fusion other through cartilaginous intervertebral joints between the is initiated in the neck and the sacral region of terrestrial centra and synovial joints between the pre- and post- mammals, while in recent aquatic mammals epiphyseal fusion zygapophyses. The mobility of each vertebral joint varies is initiated in the neck and caudal regions, suggesting loco- greatly between species as well as along the vertebral column motor pattern and environment affect fusion pattern. We also within a single species. Vertebral column mobility greatly studied bony fusion of the sacrum and evaluated criteria used impacts locomotor style, whether the animal is terrestrial or to homologize cetacean vertebrae with the fused sacrum of aquatic. In aquatic Cetacea, buoyancy counteracts gravity, and terrestrial mammals. We found that the initial ossification of the tail is the main propulsive organ (Fish 1996;Fishetal. -

Carpals and Tarsals of Mule Deer, Black Bear and Human: an Osteology Guide for the Archaeologist
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2009 Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist Tamela S. Smart Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Anthropology Commons Recommended Citation Smart, Tamela S., "Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist" (2009). WWU Graduate School Collection. 19. https://cedar.wwu.edu/wwuet/19 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. MASTER'S THESIS In presenting this thesis in partial fulfillment of the requirements for a master's degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWu. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. I acknowledge that I retain ownership rights to the copyright of this work, including but not limited to the right to use all or part of this work in future works, such as articles or books. -
Upper Limb Development
Upper Limb Development Alphonsus Chong Department of Hand and Reconstructive Microsurgery National University Hospital Why bother? Most congenital limb anomalies are due to: Disorders of embryogenesis or Problems during fetal development Some terminology Embryogenesis 0-8 weeks – new organ systems appear Fetal period Appearance of primary ossification center in humerus Differentiation, maturation and enlargement of existing organs Limb Development Limb Patterning Tissue Differentiation Why is it an arm and not Skeletal a leg? Joint Vascular Nerve Muscle and Tendon Positional Information and Axes of the upper limb Limb Bud in E3 Chick Embryo Limb bud (lateral plate) Loose mesenchymal cells from lateral plate mesoderm Ectodermal epithelial cells Migrating cells Somites --> Muscle Nerves Vasculature Limb Bud Development Limb bud Ectoderm and mesenchyme Not fully differentiated yet but all ingredients there If transplanted ectopic limb Limb Bud Regions AER Progress zone Zone of polarizing activity AER – Proximal to Distal formation Zone of Polarizing Actvity – AP development Morphogen Gradient Model Dorsal / ventral patterning less well understood Separation of Digits Apoptosis (Programmed cell death) of interdigital mesenchyme BMPs important Starts post-axial to pre-axial Mesoderm specifies amount of apoptosis How does this relate to pathogensis? Picture from Greene Learning Points UE development occurs early in embryogenesis – most risk of development congenital anomalies Pattern of limb development follows a body plan Digit formation is by apoptosis Thank You Further Reading Principles of Development 3rd Ed by Lewis Wolpert. Oxford University Press Growing Hand. Amit Gupta and Louisville Group.