Congenital Bone Deformities and the Inbred Wolves (Canis Lupus) of Isle Royale
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Copyrighted Material
C01 10/31/2017 11:23:53 Page 1 1 1 The Normal Anatomy of the Neck David Bainbridge Introduction component’ of the neck is a common site of pathology, and the diverse forms of neck The neck is a common derived characteristic disease reflect the sometimes complex and of land vertebrates, not shared by their aquatic conflicting regional variations and functional ancestors. In fish, the thoracic fin girdle, the constraints so evident in this region [2]. precursor of the scapula, coracoid and clavi- Unlike the abdomen and thorax, there is no cle, is frequently fused to the caudal aspect of coelomic cavity in the neck, yet its ventral part the skull. In contrast, as vertebrates emerged is taken up by a relatively small ‘visceral on to the dry land, the forelimb separated from compartment’, containing the larynx, trachea, the head and the intervening vertebrae speci- oesophagus and many important vessels, alised to form a relatively mobile region – the nerves and endocrine glands. However, I neck – to allow the head to be freely steered in will not review these structures, as they do many directions. not represent an extension of the equine ‘back’ With the exception of the tail, the neck in the same way that the more dorsal locomo- remains the most mobile region of the spinal tor region does. column in modern-day horses. It permits a wide range of sagittal plane flexion and exten- sion to allow alternating periods of grazing Cervical Vertebrae 3–7 and predator surveillance, as well as frontal plane flexion to allow the horizon to be scan- Almost all mammals, including the horse, ned, and rotational movement to allow possess seven cervical vertebrae, C1 to C7 nuisance insects to be flicked off. -
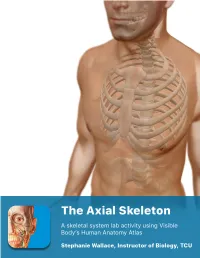
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Vertebral Column
Vertebral Column • Backbone consists of Cervical 26 vertebrae. • Five vertebral regions – Cervical vertebrae (7) Thoracic in the neck. – Thoracic vertebrae (12) in the thorax. – Lumbar vertebrae (5) in the lower back. Lumbar – Sacrum (5, fused). – Coccyx (4, fused). Sacrum Coccyx Scoliosis Lordosis Kyphosis Atlas (C1) Posterior tubercle Vertebral foramen Tubercle for transverse ligament Superior articular facet Transverse Transverse process foramen Facet for dens Anterior tubercle • Atlas- ring of bone, superior facets for occipital condyles. – Nodding movement signifies “yes”. Axis (C2) Spinous process Lamina Vertebral foramen Transverse foramen Transverse process Superior articular facet Odontoid process (dens) •Axis- dens or odontoid process is body of atlas. – Pivotal movement signifies “no”. Typical Cervical Vertebra (C3-C7) • Smaller bodies • Larger spinal canal • Transverse processes –Shorter – Transverse foramen for vertebral artery • Spinous processes of C2 to C6 often bifid • 1st and 2nd cervical vertebrae are unique – Atlas & axis Typical Cervical Vertebra Spinous process (bifid) Lamina Vertebral foramen Inferior articular process Superior articular process Transverse foramen Pedicle Transverse process Body Thoracic Vertebrae (T1-T12) • Larger and stronger bodies • Longer transverse & spinous processes • Demifacets on body for head of rib • Facets on transverse processes (T1-T10) for tubercle of rib Thoracic Vertebra- superior view Spinous process Transverse process Facet for tubercle of rib Lamina Superior articular process -

Visible Body® Human Anatomy Atlas 2021
Ovid® Visible Body® Human Anatomy Atlas 2021 New physiology content, more detailed gross anatomy, 3D INTERACTIVE navigation enhancements, user accounts, and more! ANATOMICAL STRUCTURES FOR THE Visible Body Human Anatomy Atlas 2021 goes further than previous editions in encouraging students, faculty, clinicians, and research scientists to MALE AND FEMALE explore the human body—male and female—from head to toe. Thousands of HUMAN BODY interactive anatomical structures demonstrate in vivid, accurate detail how organs, muscle, bone, and tendons move and interact. Optimized for both web and mobile access, the Atlas allows users to dissect and peel away layers, rotate models—and now draw around and annotate models for your students and colleagues. See a more complete list of enhancements to the 2021 edition below. NEW! Physiology content • Over 90 animations for patient education and advanced learning: interactions and processes depicted include cellular respiration, heart conduction, peristalsis, filtration, coronary artery disease, kidney stones, and sciatica • More than 20 advanced animations that demonstrate normal physiology conditions in much greater detail More detailed gross anatomy content • New peritoneum and organs in context help with clinical evaluation and exam prep • Improved muscle actions: muscles gray-out when they are not performing their action for clarity • Enhanced pericardium model • More detailed blood supply and innervation in female reproductive anatomy New search and navigation functionality • Search faster and move -

Cervical Vertebrae 1 Cervical Vertebrae
Cervical vertebrae 1 Cervical vertebrae Cervical vertebrae or Cervilar Position of human cervical vertebrae (shown in red). It consists of 7 bones, from top to bottom, C1, C2, C3, C4, C5, C6 and C7. A human cervical vertebra Latin Vertebrae cervicales [1] Gray's p.97 [2] MeSH Cervical+vertebrae [3] TA A02.2.02.001 [4] FMA FMA:72063 In vertebrates, cervical vertebrae (singular: vertebra) are those vertebrae immediately inferior to the skull. Thoracic vertebrae in all mammalian species are defined as those vertebrae that also carry a pair of ribs, and lie caudal to the cervical vertebrae. Further caudally follow the lumbar vertebrae, which also belong to the trunk, but do not carry ribs. In reptiles, all trunk vertebrae carry ribs and are called dorsal vertebrae. In many species, though not in mammals, the cervical vertebrae bear ribs. In many other groups, such as lizards and saurischian dinosaurs, the cervical ribs are large; in birds, they are small and completely fused to the vertebrae. The transverse processes of mammals are homologous to the cervical ribs of other amniotes. Cervical vertebrae 2 In humans, cervical vertebrae are the smallest of the true vertebrae, and can be readily distinguished from those of the thoracic or lumbar regions by the presence of a foramen (hole) in each transverse process, through which passes the vertebral artery. The remainder of this article focuses upon human anatomy. Structure By convention, the cervical vertebrae are numbered, with the first one (C1) located closest to the skull and higher numbered vertebrae (C2-C7) proceeding away from the skull and down the spine. -
Hand Bone Age: a Digital Atlas of Skeletal Maturity
V. Gilsanz/O. Ratib · Hand Bone Age Vicente Gilsanz · Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity With 88 Figures Vicente Gilsanz, M.D., Ph.D. Department of Radiology Childrens Hospital Los Angeles 4650 Sunset Blvd., MS#81 Los Angeles, CA 90027 Osman Ratib, M.D., Ph.D. Department of Radiology David Geffen School of Medicine at UCLA 100 Medical Plaza Los Angeles, CA 90095 This eBook does not include ancillary media that was packaged with the printed version of the book. ISBN 3-540-20951-4 Springer-Verlag Berlin Heidelberg New York Library of Congress Control Number: 2004114078 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable to prosecution under the German Copyright Law. Springer-Verlag Berlin Heidelberg New York Springer is a part of Springer Science+Business Media http://www.springeronline.com A Springer-Verlag Berlin Heidelberg 2005 Printed in Germany The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from therelevantprotectivelawsandregulationsandthereforefreeforgeneraluse. Product liability: The publishers cannot guarantee the accuracy of any information about the application of operative techniques and medications contained in this book. -

Hand Bone Age: a Digital Atlas of Skeletal Maturity, Second Edition
Hand Bone Age Vicente Gilsanz • Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity Second Edition Prof. Vicente Gilsanz Prof. Osman Ratib Department of Radiology University Hospital of Geneva Childrens Hospital Los Angeles Nuclear Medicine Division 4650 Sunset Blvd., MS#81 24, Rue Micheli-du-Crest Los Angeles, CA 90027 1205 Geneva USA Switzerland ISBN 978-3-642-23761-4 e-ISBN 978-3-642-23762-1 DOI 10.1007/978-3-642-23762-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2011940860 © Springer-Verlag Berlin Heidelberg 2012 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protec- tive laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and application contained in this book. In every individual case the user must check such information by consulting the relevant literature. -

Bones of the Trunk
BONES OF THE TRUNK Andrea Heinzlmann Veterinary University Department of Anatomy and Histology 16th September 2019 VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) • the vertebral column composed of the vertebrae • the vertebrae form a horizontal chain https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) along the vertebral column three major curvatures are recognized: 1. the DORSAL CONVEX CURVATURE – between the head and the neck 2. the DORSAL CONCAVE CURVATURE – between the neck and the chest 3. the DORSAL CONVEX CURVATURE – between the thorax and the lumbar region - in carnivores (Ca) there is an additional DORSAL CONVEXITY in the sacral region https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) - corresponding to the regions of the body, we distinguish: 1. CERVICAL VERTEBRAE 2. THORACIC VERTEBRAE 3. LUMBAR VERTEBRAE 4. SACRAL VERTEBRAE 5. CAUDAL (COCCYGEAL) VERTEBRAE https://www.ufaw.org.uk/dogs/french-bulldog-hemivertebrae https://rogueshock.com/know-your-horse-in-9-ways/5/ BUILD OF THE VERTEBRAE each vertebrae presents: 1. BODY (CORPUS VERTEBRAE) 2. ARCH (ARCUS VERTEBRAE) 3. PROCESSES corpus Vertebra thoracica (Th13) , Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) - the ventral portion of the vertebra ITS PARTS: 1. EXTREMITAS CRANIALIS (seu CAPUT VERTEBRAE) – convex 2. EXTREMITAS CAUDALIS (seu FOSSA VERTEBRAE) - concave Th13, Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) 3. VENTRAL SURFACE of the body has a: - ventral crest (CRISTA VENTRALIS) 4. DORSAL SURFACE of the body carries : - the vertebral arch (ARCUS VERTEBRAE) Th13, Ca., lateral aspect Arcus vertebrae corpus Vertebra thoracica (Th13) , Ca., caudal aspect THE VERTEBRAL BODY (CORPUS VERTEBRAE) 6. VERTEBRAL ARCH (ARCUS VERTEBRAE) compraisis: a) a ventral PEDICULUS ARCUS VERTEBRAE b) a dorsal LAMINA ARCUS VERTEBRAE C7, Ca. -

Morphological Study of the Foramen Transversarium of the Atlas Vertebra Among Egyptian Population and Its Clinical Significance
Research Article Anatomy Physiol Biochem Int J Volume 4 Issue 4- March 2018 Copyright © All rights are reserved by Joseph N Aziz DOI: 10.19080/APBIJ.2018.04.555642 Morphological Study of the Foramen Transversarium of the Atlas Vertebra among Egyptian Population and Its Clinical Significance Joseph N Aziz* and Michel Morgan Department of Anatomy, Cairo University, Egypt Submission: February 20, 2018; Published: March 15, 2018 *Corresponding author: Joseph N Aziz, Department of Anatomy, Faculty of Medicine, Cairo University, Egypt, Email: Abstract Background: Foramina transversaria are characteristic bony features of the cervical vertebra, they are located on the transverse process of cervical vertebrae. These foramina are of anatomical importance as they provide bony passages for several anatomical structures namely vertebral artery, vertebral vein and sympathetic nerves. They have known to exhibit variations with regard to size, shape and may even absent, incomplete or duplicated. Objective: This study aim to investigate the morphology and variations of foramina transversaria of the human atlas vertebrae and to point out the clinical importance of these variations. Material and methods: 135 atlas vertebrae of Egyptian origin were studied. They were available in the dissecting room of the anatomy department, Faculty of Medicine, Cairo University. Each vertebra was studied morphologically for the presence of various shapes of foramen transversarium, presence or absences of any morphological anomaly like accessory foramen or incomplete foramen. Results: Four shapes were noted. Type 1 (rounded) was predominant 54.1%, type 2 (oval) less prominent 29.6%, type 3 (irregular) 10.4% and type 4 (quadrangular) 5.8%. Double foramina were founded in 23 vertebras, incomplete foramina in 9 vertebras and accessory incomplete foramina were seen in 12 vertebras. -

General Anatomy and Musculoskeletal System (THIEME
TITLE General Anatomy and Musculoskeletal System (THIEME Atlas of Anatomy) 3rd Edition, Volume 1 PRICE €69.99 ISBN 9781626237186 (Previous Edition: 9781604069228, 2014) PUBLICATION DATE February 2020 FORMAT Softcover · 2074 Illustrations · 636 Pages · 9.000 X 12.250 IN MEDIA CONTENT Complimentary MedOne eBook SPECIALTY Anatomy LEVEL Medical and allied health students in MSK courses; PT students and instructors; practicing physical therapists and related professions (massage, yoga, etc.) EDITORS Michael Schuenke, MD, PhD, is Professor, Institute of Anatomy, Christian Albrecht University, Kiel, Germany. Erik Schulte, MD, is Professor, Department of Anatomy and Cell Biology, Johannes Gutenberg University, Mainz, Germany. Udo Schumacher, MD, FRCPath, CBiol, FIBiol, DSc, is Professor, Institute of Anatomy II: Experimental Morphology, University Medical Center, Hamburg-Eppendorf, Germany Nathan F. Johnson, PT, DPT, PhD, is Assistant Professor, Division of Physical Therapy, University of Kentucky College of Health Sciences, Lexington, Kentucky, USA. DESCRIPTION Remarkable atlas provides exceptionally detailed, clinically relevant anatomic knowledge! Praise for the prior edition: "This book is an ideal text not only for students of various disciplines studying anatomy for the first time, but it also serves as a valuable resource for faculty and providers."—Yale Journal of Biology and Medicine Thieme Atlas of Anatomy: General Anatomy and Musculoskeletal System, Third Edition by renowned educators Michael Schuenke, Erik Schulte, and Udo Schumacher, along with consulting editor Nathan Johnson, expands on the award-winning prior editions with updated spreads and added information on joints, muscle actions, and functional muscle groups. Organized by region, the book begins with an introduction on basic human embryology and development and an overview of the human body. -

Lab Exercise 5 Axial Skeleton
Lab Exercise 5 Axial Skeleton Textbook Reference: See Chapter 8 What you need to be able to do on the exam after completing this lab exercise: Be able to name all the listed bone and bone features on the skull models. Be able to name the listed parts of the ribcage and sternum on the models in the lab. Be able to name the hyoid bone, if shown individually. Be able to name the different vertebrae, if shown individually, as given in this lab exercise. Be able to name the listed features of each vertebra on the vertebrae in the lab. Be able to name the sacrum and the listed parts of the sacrum on the models in the lab. Be able to name the coccyx, if individually shown. 5-1 The following tables contain terms that are useful when learning the various bone features. The terms will NOT be on the test. They are simply here for you to use when learning the names of the bone features. 5 -2 Axial Skeleton The axial skeleton consists of the skull, the ribcage, and the vertebrae. The Skull Know the following bones/bone features on the skull models. 1. frontal bone 10. sphenoid bone 21. coronoid process 2. parietal bone 11. zygomatic bone 22. mandibular condyle 3. occipital bone 12. nasal bone 23. coronal suture 4. temporal bone 13. maxilla 25. lambdoid suture 5. mastoid process 14. lacrimal bone 28. squamous suture 8. mandibular fossa 15. ethmoid bone 42. external auditory meatus 9. styloid process 20. mandible 52. mental foramen 62. -

1. Anatomical Basis of Thoracic Surgery
BWH 2015 GENERAL SURGERY RESIDENCY PROCEDURAL ANATOMY COURSE 1. ANATOMICAL BASIS OF THORACIC SURGERY Contents Lab objectives ............................................................................................................................................... 2 Knowledge objectives ............................................................................................................................... 2 Skills objectives ......................................................................................................................................... 2 Preparation for lab ....................................................................................................................................... 2 1.1 BASIC PRINCIPLES OF ANATOMICAL ORGANIZATION ............................................................................ 4 1.2 THORACIC CAVITY AND CHEST WALL ..................................................................................................... 9 1.3 PLEURA AND LUNGS ............................................................................................................................. 13 1.4 ORGANIZATION OF THE MEDIASTINUM .............................................................................................. 19 1.5 ANTERIOR mediastinum ....................................................................................................................... 23 Thymus ...................................................................................................................................................