Hand Bone Age: a Digital Atlas of Skeletal Maturity, Second Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association Between Dietary Habits and Parental Health with Obesity Among Children with Precocious Puberty
children Article Association between Dietary Habits and Parental Health with Obesity among Children with Precocious Puberty 1, 1, 2 1 3, Yong Hee Hong y , Yeon Ju Woo y, Jong Hyun Lee , Young-Lim Shin and Hee-Sook Lim * 1 Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University School of Medicine, Bucheon 14584, Korea; [email protected] (Y.H.H.); [email protected] (Y.J.W.); [email protected] (Y.-L.S.) 2 Department of Pediatrics, Soonchunhyang University Gumi Hospital, Soonchunhyang University School of Medicine, Gumi 39371, Korea; [email protected] 3 Department of Food Sciences and Nutrition, Yeonsung University, Anyang 14011, Korea * Correspondence: [email protected] These authors contributed equally to this work as first author. y Received: 25 September 2020; Accepted: 5 November 2020; Published: 8 November 2020 Abstract: Precocious puberty, resulting in various physical, mental, and social changes, may have negative consequences for children and their families. In this study, we investigated whether there were differences between parental obesity, children’s and parent’s awareness of body shape, and dietary habits according to obesity levels in children with precocious puberty. A total of 193 children (93.3% girls) diagnosed with precocious puberty were classified into three groups according to their obesity levels. Negative body shape awareness and dissatisfaction were significantly higher in the obese group than in the normal-weight group, and parents were more likely to perceive their children as fat than the children themselves. In addition, the obesity rate of parents in the obese group was higher, and the body mass indexes of children and parents were significantly correlated. -

Copyrighted Material
C01 10/31/2017 11:23:53 Page 1 1 1 The Normal Anatomy of the Neck David Bainbridge Introduction component’ of the neck is a common site of pathology, and the diverse forms of neck The neck is a common derived characteristic disease reflect the sometimes complex and of land vertebrates, not shared by their aquatic conflicting regional variations and functional ancestors. In fish, the thoracic fin girdle, the constraints so evident in this region [2]. precursor of the scapula, coracoid and clavi- Unlike the abdomen and thorax, there is no cle, is frequently fused to the caudal aspect of coelomic cavity in the neck, yet its ventral part the skull. In contrast, as vertebrates emerged is taken up by a relatively small ‘visceral on to the dry land, the forelimb separated from compartment’, containing the larynx, trachea, the head and the intervening vertebrae speci- oesophagus and many important vessels, alised to form a relatively mobile region – the nerves and endocrine glands. However, I neck – to allow the head to be freely steered in will not review these structures, as they do many directions. not represent an extension of the equine ‘back’ With the exception of the tail, the neck in the same way that the more dorsal locomo- remains the most mobile region of the spinal tor region does. column in modern-day horses. It permits a wide range of sagittal plane flexion and exten- sion to allow alternating periods of grazing Cervical Vertebrae 3–7 and predator surveillance, as well as frontal plane flexion to allow the horizon to be scan- Almost all mammals, including the horse, ned, and rotational movement to allow possess seven cervical vertebrae, C1 to C7 nuisance insects to be flicked off. -

Meyer Dysplasia in the Differential Diagnosis of Hip Disease in Young Children
ARTICLE Meyer Dysplasia in the Differential Diagnosis of Hip Disease in Young Children Liora Harel, MD; Liora Kornreich, MD; Shai Ashkenazi, MD, MSc; Avinoam Rachmel, MD; Boaz Karmazyn, MD; Jacob Amir, MD Objectives: To describe a rare developmental disorder Results: Two of the 5 patients were initially diagnosed of the femoral capital epiphysis in infants and children as having osteomyelitis and 3 as having Perthes disease. that is often misdiagnosed and to suggest an evaluation The diagnosis of Meyer dysplasia was confirmed by plain protocol to differentiate it from other hip problems. film of the pelvis, a negative bone scan, or normal bone marrow findings on magnetic resonance imaging. The Design: Case series. limping resolved without treatment in all patients within 1 to 3 weeks. Setting: Tertiary care center. Conclusions: Meyer dysplasia is a benign condition that Subjects: Five consecutive patients referred for evalu- should be included in the differential diagnosis of hip dis- ation of acute onset of limping between January 1990 and ease in infants and children. Awareness of this condi- December 1997. tion may prevent unnecessary hospitalization and treat- ment. Intervention: All clinical and imaging data were col- lected. Arch Pediatr Adolesc Med. 1999;153:942-945 5 patients. The 5 children included 4 males Editor’s Note: Keep this in mind next time you see a limping and 1 female aged 9 to 48 months. All pre- toddler or preschooler. sented with acute onset of limping of 2 to Catherine D. DeAngelis, MD 30 days’ duration. Patient 3 was being ob- served by a pediatric endocrinologist for short stature, and patient 4 had a family EYER dysplasia is a history of congenital dislocation of the hip symptomless develop- and Perthes disease. -

Identification of Teeth, Wrist and Femur Bone Features for Age and Gender Identifiction
Vidyashree H S et al. / International Journal of Computer Science Engineering (IJCSE) IDENTIFICATION OF TEETH, WRIST AND FEMUR BONE FEATURES FOR AGE AND GENDER IDENTIFICTION Vidyashree H S Department of Computer Science and Engineering, Bapuji Institute of Engineering and Technology, Davanagere, India. Email: [email protected] Pradeep N Department of Computer Science and Engineering, Bapuji Institute of Engineering and Technology, Davanagere, India. Email: [email protected] Abstract: Personal identification is defined as establishing the identity of an individual. The need for personal identification arises in natural mass disasters like earth quakes, tsunamis, landslides, floods etc., and in man-made disasters such as terrorist attacks, bomb blasts, mass murders, and in cases when the body is highly decomposed or dismembered to deliberately conceal the identity of the individual. Computers have been widely used in the field of medical research over the past few decades. Machine Learning and Biomedical Image Processing Techniques have enormously contributed in the field of Medical Image Analysis, classification and recognition. But fewer contributions have been made in the area of forensics by researchers. There is a scope for researches, where they can make their contributions especially in medical image analysis where they can estimate age and identify gender using digital X-ray images. In this paper, we are identifying features of Femur, Teeth and Wrist features that are helpful for age and gender identification. Keywords- Forensic Science, Femur Bone Age, Gender Estimation, teeth, wrist bone age and gender comparison. I.INTRODUCTION The age of an individual is often a fundamental piece of data in connection with forensic identification of unidentified bodies. -

Original Article Clinical Characteristics and Mutation Analysis of Two Chinese Children with 17A-Hydroxylase/17,20-Lyase Deficiency
Int J Clin Exp Med 2015;8(10):19132-19137 www.ijcem.com /ISSN:1940-5901/IJCEM0013391 Original Article Clinical characteristics and mutation analysis of two Chinese children with 17a-hydroxylase/17,20-lyase deficiency Ziyang Zhu, Shining Ni, Wei Gu Department of Endocrinology, Nanjing Children’s Hospital Affiliated to Nanjing Medical University, Nanjing 210008, China Received July 25, 2015; Accepted September 10, 2015; Epub October 15, 2015; Published October 30, 2015 Abstract: Combined with the literature, recognize the clinical features and molecular genetic mechanism of the disease. 17a-hydroxylase/17,20-lyase deficiency, a rare form of congenital adrenal hyperplasia, is caused by muta- tions in the cytochrome P450c17 gene (CYP17A1), and characterized by hypertension, hypokalemia, female sexual infantilism or male pseudohermaphroditism. We presented the clinical and biochemical characterization in two patients (a 13 year-old girl (46, XX) with hypokalemia and lack of pubertal development, a 11 year-old girl (46, XY) with female external genitalia and severe hypertension). CYP17A1 mutations were detected by PCR and direct DNA sequencing in patients and their parents. A homozygous mutation c.985_987delTACinsAA (p.Y329KfsX418) in Exon 6 was found in patient 1, and a homozygous deletion mutation c.1459_1467delGACTCTTTC (p.Asp487_Phe489del) in exon 8 in patient 2. The patients manifested with hypertension, hypokalemia, sexual infantilism should be sus- pected of having 17a-hydroxylase/17,20-lyase deficiency. Definite diagnosis is depended on mutation analysis. Hydrocortisone treatment in time is crucial to prevent severe hypertension and hypokalemia. Keywords: 17a-hydroxylase/17,20-lyase deficiency Introduction patients with 17a-hydroxylase/17,20-lyase defi- ciency, and made a confirmative diagnosis by Deficiency in cytochrome p450c17 (MIM mutation analysis of CYP17A1. -

Alan E. Oestreich Growth of the Pediatric Skeleton a Primer for Radiologists Alan E
Alan E. Oestreich Growth of the Pediatric Skeleton A Primer for Radiologists Alan E. Oestreich Growth of the Pediatric Skeleton A Primer for Radiologists With illustrations by Tamar Kahane Oestreich 123 Alan E. Oestreich, MD, FACR Radiology 5031 Cincinnati Children’s Hospital Medical Center 3333 Burnett Ave. Cincinnati OH 45229-3039 USA Library of Congress Control Number: 2007933312 ISBN 978-3-540-37688-0 Springer Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is con- cerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitations, broadcasting, reproduction on microfi lm or in any other way, and storage in data banks. Duplication of this publica- tion or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law. Springer is part of Springer Science+Business Media http//www.springer.com Springer-Verlag Berlin Heidelberg 2008 Printed in Germany The use of general descriptive names, trademarks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regula- tions and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and application contained in this book. In every case the user must check such information by consulting the relevant literature. Medical Editor: Dr. -

REVIEW ARTICLE Interactions Between GH, IGF-I, Glucocorticoids
0031-3998/02/5202-0137 PEDIATRIC RESEARCH Vol. 52, No. 2, 2002 Copyright © 2002 International Pediatric Research Foundation, Inc. Printed in U.S.A. REVIEW ARTICLE Interactions between GH, IGF-I, Glucocorticoids, and Thyroid Hormones during Skeletal Growth HELEN ROBSON, THOMAS SIEBLER, STEPHEN M. SHALET, AND GRAHAM R. WILLIAMS Department of Clinical Research [H.R.], Department of Endocrinology [S.M.S.], Christie Hospital National Health Service Trust, Manchester, U.K.; University Children’s Hospital, University of Leipzig, Leipzig, Germany [T.S.]; IC Molecular Endocrinology Group, Division of Medicine and MRC Clinical Sciences Centre, Faculty of Medicine, Imperial College School of Science Technology and Medicine, Hammersmith Hospital, London, U.K. [G.R.W.] ABSTRACT Linear growth occurs during development and the childhood Abbreviations years until epiphyseal fusion occurs. This process results from 5'-DI, 5'-iodothyronine deiodinase endochondral ossification in the growth plates of long bones and FGF, fibroblast growth factor is regulated by systemic hormones and paracrine or autocrine FGFR, fibroblast growth factor receptor factors. The major regulators of developmental and childhood GC, glucocorticoid growth are GH, IGF-I, glucocorticoids, and thyroid hormone. GHR, GH receptor Sex steroids are responsible for the pubertal growth spurt and GR, glucocorticoid receptor epiphyseal fusion. This review will consider interactions between HSPG, heparan sulfate proteoglycan GH, IGF-I, glucocorticoids, and thyroid hormone during linear 11HSD, 11-hydroxysteroid dehydrogenase growth. It is well known from physiologic and clinical studies IGF-IR, IGF-I receptor that these hormones interact at the level of the hypothalamus and IGFBP, IGF binding protein pituitary. Interacting effects on peripheral tissues such as liver are Ihh, Indian hedgehog also well understood, but we concentrate here on the epiphyseal JAK-2, Janus-activated kinase-2 growth plate as an important and newly appreciated target organ PTHrP, PTH-related peptide for convergent hormone action. -

Guidelines for Best Practice Imaging for Age Estimation in the Living
Journal of Forensic Radiology and Imaging 16 (2019) 38–49 Contents lists available at ScienceDirect Journal of Forensic Radiology and Imaging journal homepage: www.elsevier.com/locate/jofri Guidelines for best practice: Imaging for age estimation in the living T ⁎ Edel Doylea, , Nicholas Márquez-Grantb, Lisa Fieldc, Trish Holmesa, Owen J Arthursd,e,f, Rick R. van Rijng,h, Lucina Hackmani, Kathleen Kasperj, Jim Lewisk,l, Peter Loomism, Denise Elliotta, Jeroen Krolla,n, Mark Vinera,b,o, Soren Blaup, Alison Broughq,r, Stella Martín de las Herass, Pedro Manuel Garamendit,u,v a International Association of Forensic Radiographers, UK b Cranfield Forensic Institute, Cranfield University, Defence Academy of the United Kingdom,UK c International Society of Radiographers & Radiologic Technologists, UK d Department of Radiology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, UK e UCL Great Ormond Street Institute of Child Health, London, UK f ISFRI Paediatric Working Group, Switzerland g Department of Radiology, Emma Children's Hospital – Academic Medical Center Amsterdam, the Netherlands h Department of Forensic Medicine, Netherlands Forensic Institute, The Hague, the Netherlands i Leverhulme Centre for Forensic Science, Ewing Building, University of Dundee, Dundee, UK j Tarrant County Medical Examiner's District, Ft. Worth, Texas; Forensic Odontologist, Collin County Medical Examiner, McKinney, TX, USA k The University of Tennessee, Graduate School of Medicine, Department of General Dentistry, Division of Forensic Odontology, -

Congenital Bone Deformities and the Inbred Wolves (Canis Lupus) of Isle Royale
ARTICLE IN PRESS Biological Conservation xxx (2009) xxx–xxx Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Congenital bone deformities and the inbred wolves (Canis lupus) of Isle Royale Jannikke Räikkönen a,*, John A. Vucetich b, Rolf O. Peterson b, Michael P. Nelson c a Swedish Museum of Natural History, Department of Contaminant Research, Frescativägen 44, P.O. Box 50007, S-104 05 Stockholm, Sweden b School of Forest Resources and Environmental Science, Michigan Technological University, Houghton, MI 49931, USA c Lyman Briggs College, Department of Fisheries and Wildlife, and Department of Philosophy, Michigan State University, East Lansing, MI 48825, USA a r t i c l e i n f o a b s t r a c t Article history: The wolf (Canis lupus) population on Isle Royale, a remote island in Lake Superior, North America, is extre- Received 26 October 2008 mely inbred. Nevertheless, the consequences of genetic deterioration have not been detected for this Received in revised form 21 January 2009 intensively studied population, until now. We found that 58% (n = 36) of Isle Royale wolves exhibited Accepted 24 January 2009 some kind of congenital malformation in the lumbosacral region of the vertebral column and 33% exhib- Available online xxxx ited a specific malformity, lumbosacral transitional vertebrae. By contrast, only 1% (1 of 99) of wolves sampled from two outbred, wolf populations exhibited this malformity. Moreover, in domestic dogs Keywords: (Canis lupus familiaris) lumbosacral transitional vertebrae are associated with cauda equina syndrome, Canis lupus which can cause paresis, paralysis, locomotor difficulties in the rear legs and tail, and back pain. -
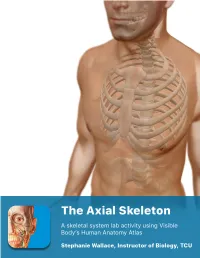
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Vertebral Column
Vertebral Column • Backbone consists of Cervical 26 vertebrae. • Five vertebral regions – Cervical vertebrae (7) Thoracic in the neck. – Thoracic vertebrae (12) in the thorax. – Lumbar vertebrae (5) in the lower back. Lumbar – Sacrum (5, fused). – Coccyx (4, fused). Sacrum Coccyx Scoliosis Lordosis Kyphosis Atlas (C1) Posterior tubercle Vertebral foramen Tubercle for transverse ligament Superior articular facet Transverse Transverse process foramen Facet for dens Anterior tubercle • Atlas- ring of bone, superior facets for occipital condyles. – Nodding movement signifies “yes”. Axis (C2) Spinous process Lamina Vertebral foramen Transverse foramen Transverse process Superior articular facet Odontoid process (dens) •Axis- dens or odontoid process is body of atlas. – Pivotal movement signifies “no”. Typical Cervical Vertebra (C3-C7) • Smaller bodies • Larger spinal canal • Transverse processes –Shorter – Transverse foramen for vertebral artery • Spinous processes of C2 to C6 often bifid • 1st and 2nd cervical vertebrae are unique – Atlas & axis Typical Cervical Vertebra Spinous process (bifid) Lamina Vertebral foramen Inferior articular process Superior articular process Transverse foramen Pedicle Transverse process Body Thoracic Vertebrae (T1-T12) • Larger and stronger bodies • Longer transverse & spinous processes • Demifacets on body for head of rib • Facets on transverse processes (T1-T10) for tubercle of rib Thoracic Vertebra- superior view Spinous process Transverse process Facet for tubercle of rib Lamina Superior articular process -

Visible Body® Human Anatomy Atlas 2021
Ovid® Visible Body® Human Anatomy Atlas 2021 New physiology content, more detailed gross anatomy, 3D INTERACTIVE navigation enhancements, user accounts, and more! ANATOMICAL STRUCTURES FOR THE Visible Body Human Anatomy Atlas 2021 goes further than previous editions in encouraging students, faculty, clinicians, and research scientists to MALE AND FEMALE explore the human body—male and female—from head to toe. Thousands of HUMAN BODY interactive anatomical structures demonstrate in vivid, accurate detail how organs, muscle, bone, and tendons move and interact. Optimized for both web and mobile access, the Atlas allows users to dissect and peel away layers, rotate models—and now draw around and annotate models for your students and colleagues. See a more complete list of enhancements to the 2021 edition below. NEW! Physiology content • Over 90 animations for patient education and advanced learning: interactions and processes depicted include cellular respiration, heart conduction, peristalsis, filtration, coronary artery disease, kidney stones, and sciatica • More than 20 advanced animations that demonstrate normal physiology conditions in much greater detail More detailed gross anatomy content • New peritoneum and organs in context help with clinical evaluation and exam prep • Improved muscle actions: muscles gray-out when they are not performing their action for clarity • Enhanced pericardium model • More detailed blood supply and innervation in female reproductive anatomy New search and navigation functionality • Search faster and move