Addis Ababa University Institute of Biotechnology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Ermias Bonkola
St. MARY’S UNIVERSITY COLLEGE FACULTY OF BUSINESS DEPARTMENT OF MANAGEMENT ASSESSMENT OF TRENDS OF HUMAN RESOURCES MANAGEMENT: THE CASE OF MISHA WOREDA, HADIYA ZONE, SNNPR, ETHIOPIA BY ERMIAS BONKOLA A SENIOR ESSAY PAPER SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF BACHELOR OF ARTS IN MANAGEMENT MARCH 2013 SMUC St. MARY’S UNIVERSITY COLLEG FACULTY OF BUSINESS DEPARTEMENT OF MANAGEMENT This is to certify that the senor essay prepared by Ermias Bonkola: in partial fulfillment of the requirements for a Degree of Bachelor of Arts in Management complies with the regulations of the University college and meets the accepted standards with respect to quality. APPROVED BY THE COMMITTEE OF EXAMINERS Chair person Signature Advisor Signature Internal Examiner Signature External Examiner Signature Acknowledgement Above all, I thank Almighty God for always with me in all my endeavors and giving me endurance to complete my study. I am very glad to express my sincere gratitude and appreciation to my advisor Tamirat Sulamo (M.A) for his invaluable guidance and constructive professional advises throughout my research. Especial thanks also to my family who were always by my side and who offered me financial, the material and moral support to complete this research work as well as may study. Moreover, I would like to express my deepest gratitude to my best friend and staff members for their technical assistance and moral support in the due courses my research works and studies. Finally, I also grateful to surveyed government works and werada civil service department and data enumerators area are duly acknowledged for providing their willingness and valuable supports/cooperation. -

Demography and Health
SNNPR Southern Nations Nationalities and Peoples Demography and Health Aynalem Adugna, July 2014 www.EthioDemographyAndHealth.Org 2 SNNPR is one of the largest regions in Ethiopia, accounting for more than 10 percent of the country’s land area [1]. The mid-2008 population is estimated at nearly 16,000,000; almost a fifth of the country’s population. With less than one in tenth of its population (8.9%) living in urban areas in 2008 the region is overwhelmingly rural. "The region is divided into 13 administrative zones, 133 Woredas and 3512 Kebeles, and its capital is Awassa." [1] "The SNNPR is an extremely ethnically diverse region of Ethiopia, inhabited by more than 80 ethnic groups, of which over 45 (or 56 percent) are indigenous to the region (CSA 1996). These ethnic groups are distinguished by different languages, cultures, and socioeconomic organizations. Although none of the indigenous ethnic groups dominates the ethnic makeup of the national population, there is a considerable ethnic imbalance within the region. The largest ethnic groups in the SNNPR are the Sidama (17.6 percent), Wolayta (11.7 percent), Gurage (8.8 percent), Hadiya (8.4 percent), Selite (7.1 percent), Gamo (6.7 percent), Keffa (5.3 percent), Gedeo (4.4 percent), and Kembata (4.3 percent) …. While the Sidama are the largest ethnic group in the region, each ethnic group is numerically dominant in its respective administrative zone, and there are large minority ethnic groups in each zone. The languages spoken in the SNNPR can be classified into four linguistic families: Cushitic, Nilotic, Omotic, and Semitic. -
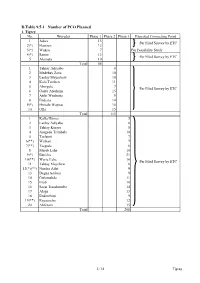
D.Table 9.5-1 Number of PCO Planned 1
D.Table 9.5-1 Number of PCO Planned 1. Tigrey No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Adwa 13 Per Filed Survey by ETC 2(*) Hawzen 12 3(*) Wukro 7 Per Feasibility Study 4(*) Samre 13 Per Filed Survey by ETC 5 Alamata 10 Total 55 1 Tahtay Adiyabo 8 2 Medebay Zana 10 3 Laelay Mayechew 10 4 Kola Temben 11 5 Abergele 7 Per Filed Survey by ETC 6 Ganta Afeshum 15 7 Atsbi Wenberta 9 8 Enderta 14 9(*) Hintalo Wajirat 16 10 Ofla 15 Total 115 1 Kafta Humer 5 2 Laelay Adiyabo 8 3 Tahtay Koraro 8 4 Asegede Tsimbela 10 5 Tselemti 7 6(**) Welkait 7 7(**) Tsegede 6 8 Mereb Lehe 10 9(*) Enticho 21 10(**) Werie Lehe 16 Per Filed Survey by ETC 11 Tahtay Maychew 8 12(*)(**) Naeder Adet 9 13 Degua temben 9 14 Gulomahda 11 15 Erob 10 16 Saesi Tsaedaemba 14 17 Alage 13 18 Endmehoni 9 19(**) Rayaazebo 12 20 Ahferom 15 Total 208 1/14 Tigrey D.Table 9.5-1 Number of PCO Planned 2. Affar No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Ayisaita 3 2 Dubti 5 Per Filed Survey by ETC 3 Chifra 2 Total 10 1(*) Mile 1 2(*) Elidar 1 3 Koneba 4 4 Berahle 4 Per Filed Survey by ETC 5 Amibara 5 6 Gewane 1 7 Ewa 1 8 Dewele 1 Total 18 1 Ere Bti 1 2 Abala 2 3 Megale 1 4 Dalul 4 5 Afdera 1 6 Awash Fentale 3 7 Dulecha 1 8 Bure Mudaytu 1 Per Filed Survey by ETC 9 Arboba Special Woreda 1 10 Aura 1 11 Teru 1 12 Yalo 1 13 Gulina 1 14 Telalak 1 15 Simurobi 1 Total 21 2/14 Affar D.Table 9.5-1 Number of PCO Planned 3. -

International Journal of Multidisciplinary Educational Research
Volume 4, Issue 1(1), January 2015 International Journal of Multidisciplinary Educational Research Published by Sucharitha Publications Visakhapatnam – 530 017 Andhra Pradesh – India Email: [email protected] Website: www.ijmer.in Editorial Board Editor-in-Chief Dr. Victor Babu Koppula Faculty, Department of Philosophy Andhra University – Visakhapatnam -530 003 Andhra Pradesh – India EDITORIAL BOARD MEMBERS Prof. S.Mahendra Dev Prof. Josef HÖCHTL Vice Chancellor Department of Political Economy Indira Gandhi Institute of Development University of Vienna, Vienna & Research Ex. Member of the Austrian Parliament Mumbai Austria Prof.Y.C. Simhadri Prof. Alexander Chumakov Vice Chancellor, Patna University Chair of Philosophy Department Former Director Russian Philosophical Society Institute of Constitutional and Parliamentary Moscow, Russia Studies, New Delhi & Formerly Vice Chancellor of Prof. Fidel Gutierrez Vivanco Benaras Hindu University, Andhra University Founder and President Nagarjuna University, Patna University Escuela Virtual de Asesoría Filosófica Lima Peru Prof. (Dr.) Sohan Raj Tater Former Vice Chancellor Prof. Igor Kondrashin Singhania University, Rajasthan The Member of The Russian Philosophical Society Prof.K.Sreerama Murty The Russian Humanist Society and Expert of Department of Economics the UNESCO, Moscow, Russia Andhra University - Visakhapatnam Dr. Zoran Vujisiæ Prof. K.R.Rajani Rector Department of Philosophy St. Gregory Nazianzen Orthodox Institute Andhra University – Visakhapatnam Universidad Rural de Guatemala, GT, U.S.A Prof. A.B.S.V.Rangarao Swami Maheshwarananda Department of Social Work Founder and President Andhra University – Visakhapatnam Shree Vishwa Deep Gurukul Swami Maheshwarananda Ashram Education Prof.S.Prasanna Sree & Research Center Department of English Rajasthan, India Andhra University – Visakhapatnam Prof.U.Shameem Prof. P.Sivunnaidu Department of Zoology Department of History Andhra University Visakhapatnam Andhra University – Visakhapatnam Dr. -

Ethiopia Social Accountability Program (ESAP3)
Ethiopia Social Accountability Program (ESAP3) Grant Agreement [TF0A9293] Progress Report Project Year 2, Quarter 3 July – September 2020 MANAGEMENT AGENCY Multi Donor Trust Fund Table of contents Table of contents ..................................................................................... 2 List of Acronyms ..................................................................................... 3 Executive Summary ............................................................................... 4 Technical Progress .................................................................................. 5 1. MA main activities .......................................................................................... 5 1.1. COTL support and coordination .......................................................................................... 5 1.2 Resumption of SA activities ................................................................................................. 5 2. SAIP activities ................................................................................................ 5 2.1 Accessing citizens in ESAP operational areas with COVID-19 information through CRs .. 8 2.1.1Dissemination of reliable, factual and up to date COVID-19 information .................... 8 2.1.2 Reaching out to community groups with special needs .............................................. 8 2.1.3 Engaging community leaders and influential citizens .................................................. 9 2.1.4 Using other innovative mechanisms to access -

Research Article
s z Available online at http://www.journalcra.com INTERNATIONAL JOURNAL OF CURRENT RESEARCH International Journal of Current Research Vol. 12, Issue, 09, pp.13881-13886, September, 2020 DOI: https://doi.org/10.24941/ijcr.39276.09.2020 ISSN: 0975-833X RESEARCH ARTICLE IMPACT OF MICROFINANCE ON LIVELIHOOD OF SMALL HOLDER FARMERS IN HADIYA ZONE, SOUTHERN ETHIOPIA Berhanu Kedir*1 and Berhanu Megerssa Beraka2 1SNNP Regional State Agricultural Department, Jimma Ethiopia 2Jimma University College of Agriculture and Veterinary Medicine, POB, 307, Jimma Ethiopia ARTICLE INFO ABSTRACT Article History: Microfinance is a fundamental tool for poverty reduction by providing financial services to low- Received 24th June, 2020 income individuals who are devoid of access to formal financial services. Microfinance institutions Received in revised form started operations in Ethiopia following P roclamation No. 40/96, and currently, there are 30 licensed 09th July, 2020 MFIs and 448 branches with active clients of 2.3 million. Despite their outstanding contributions, Accepted 14th August, 2020 th however, their ability to improve livelihood is still in question. Multi-stage sampling was used to Published online 30 September, 2020 select a representative sample size of participants and non-participants. Descriptive analysis was run to reveal measures of central tendency and variability; while logistic regression was applied to Key Words: determine factors affecting households’ decision to participate in microfinance. Logistic regression Hadiya; Livelihood; analysis indicated that seven variables significantly influenced program participation were four of them significant at 1% (i.e. Education level, household size, membership of cooperative and Microfinance; Participants. extension contact); and the other two variables were found significant at 10% (i.e. -

(SNNPR) Overview of Livelihood Profiles
Ethiopia Southern Nations, Nationalities and Peoples Region (SNNPR) Overview of Livelihood Profiles SNNPR Follow-On to Regional Livelihoods Baseline Study 2005 This document was produced for review by the United States Agency for International Development. It was prepared by Chemonics International Inc. ETHIOPIA SNNPR FOLLOW-ON TO REGIONAL LIVELIHOODS BASELINE STUDY Contract No. 663-C-00-05-00446-00 The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. SNNPR LIVELIHOOD PROFILES Introduction USAID FEWS NET PROJECT Regional Overview Contents Page INTRODUCTION........................................................................................... 1 THE USES OF THE PROFILES .................................................................... 1 KEY CONCEPTS....................................................................................... 2 INTRODUCTION TO THE HOUSEHOLD ECONOMY APPROACH................... 3 WHAT IS IN A LIVELIHOOD PROFILE........................................................ 6 METHODOLOGY ...................................................................................... 7 REGIONAL OVERVIEW............................................................................. 8 INTRODUCTION ....................................................................................... 8 GEOGRAPHY AND CLIMATE .................................................................... 9 RURAL LIVELIHOOD ZONES ................................................................... -

Somalia Livelihood Maps
Southern Nation, Nationalities and People’s Region, Ethiopia Livelihood Profiles January 2006 USAID FEWS NET ACTIVITY Contents Page INTRODUCTION........................................................................................... 1 THE USES OF THE PROFILES .................................................................... 1 KEY CONCEPTS....................................................................................... 2 INTRODUCTION TO THE HOUSEHOLD ECONOMY APPROACH................... 3 WHAT IS IN A LIVELIHOOD PROFILE........................................................ 6 METHODOLOGY ...................................................................................... 7 REGIONAL OVERVIEW............................................................................. 8 INTRODUCTION ....................................................................................... 8 GEOGRAPHY AND CLIMATE .................................................................... 9 RURAL LIVELIHOOD ZONES .................................................................... 11 RURAL SOURCES OF FOOD AND CASH: MAIN FINDINGS AND IMPLICATIONS ....................................................................... 13 RURAL LIVELIHOOD ZONE SUMMARIES.................................................. 20 Regional Overview 1 Introduction The Livelihood Profiles that follow document how the rural populations of the Southern Nations, Nationalities and Peoples’ Regional State (SNNPR) live. A livelihood is the sum of ways in which households make ends meet from -

In the Case of Soro Woreda, SNNPR, Ethiopia)
International Journal of Academic Research ISSN: 2348-7666; Vol.4, Issue-3 (1), March, 2017 Impact Factor: 4.535; Email: [email protected] Effect of Household and Environmental characteristics on the household welfare: (In the case of Soro Woreda, SNNPR, Ethiopia) Bereket Admasu, Economics department Lecturer, College of Business and Economics, Assosa University, Assosa, Ethiopia Abstract: The present study is aimed to analyze the effect of household and environment characteristics on the household welfare or poverty using primary cross- sectional data collected from six Kebeles of Soro Woreda over the sample of 251 households for the time period 2014/15. In empirical analysis researcher used the descriptive statistics to compare poverty indices (FGT poverty indices) of group of household along with household and environment characteristics and the econometric approaches (OLS and Probit regression model) to look into impact and to link with probability of being poor. Based on the analysis, this study found that the household characteristics such as household size and dependency ratio are found to be significantly and negatively affects the welfare of household whereas education, landholding and literacy ratio has a positive effect. Regarding environmental characteristics, household residence in Weina Dega and household having good soil and flat farmland has less likely of being worsened than their counterpart. Finally, the researcher mainly recommend that efforts should be made to improve possible characteristics like working more in education, family planning, and on practicing agro-ecological farming system to adopt ongoing environmental condition and to keep environment degradation in order to alleviate poverty. Key words: Household characteristics, Environmental characteristics, welfare, poverty, FGT poverty indices, effect, OLS and Probit. -

Addis Ababa University School of Graduate Studies Department of Public Admnistration and Development Management
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES DEPARTMENT OF PUBLIC ADMNISTRATION AND DEVELOPMENT MANAGEMENT THE FACTORS CONTRIBUTING FOR THE EXPANSION OF INFORMAL SETTLEMENTS IN HOSSANA TOWN, SOUTHERN ETHIOPIA BY ASHENAFI GETACHEW ANULO JUNE 2015 1 Declaration Addis Ababa University College of Business and Economics Department of Public Administration and Development Management This is to certify that the thesis prepared by Ashenafi Getachew Anulo entitled Factors for the Expansion of Informal Settlements in Hossana Town, Southern Ethiopia, which is submitted in partial fulfillment of the requirements for the degree of Master in Public Management and Policy (MPMP), complies with the University and meets the accepted standards with respect to originality. Approved by Board of Examiner: __________________________________ Signature___________ Date________________ Advisor __________________________________ Signature___________ Date________________ Internal Examiner __________________________________ Signature___________ Date________________ External Examiner __________________________________ Signature___________ Date________________ Chair of Department or Graduate Program Coordinator 2 Abstract Rapid growth of informal settlements in developing countries constitutes one of the most intriguing forms of urbanization. However, factors and theories underpinning this rapid and prevalent dynamic of informal settlements are often restricted to the descriptive phase. The purpose of this study is to investigates the land administration model -

Land Degradation and Farmers' Perception: The
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES ENVIRONMENTAL SCIENCE PROGRAM LAND DEGRADATION AND FARMERS’ PERCEPTION: THE CASE OF LIMO WOREDA, HADYA ZONE OF SNNPR, ETHIOPIA Thesis Submitted to School of Graduate Studies, Addis Ababa University, in Partial Fulfillment of the Requirements for the Attainment of the Degree of Mastersof Masters of Science in Environmental Science By Shibru Tefera Blata June, 2010 LAND DEGRADATION AND FARMERS’ PERCEPTION: THE CASE OF LIMO WOREDA, HADYA ZONE OF SNNPR, ETHIOPIA By Shibru Tefera Blata A THESIS SUBMITED TO THE SCHOOL OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE DEGREE OF MASTER OF SCIENCE IN ENVIRONMENTAL SCIENCE ENVIRONMENTAL SCIENCE PROGRAM COLLEGE OF NATURAL SCIENCE ADDIS ABABA UNIVERSITY June 2010 Addis Ababa Acknowledgements It could have been impossible to write this thesis without the assistance and contribution of many people. First and foremost I would like to thank my advisor Dr. Belay Simane for his advice, support and help with preparation and completion of this thesis. I would like to express my warm gratitude to my best friend Habitewold Ayenachew for his unreserved support especially for provision of reading materials. I would like to forward my warm appreciation to Limo Woreda Agriculture office officials, experts, development agents and staff workers for their cooperation and devotion during collection of data. I would like to thank Ato Teklu Cheru and Woizerit Genet Cheru for their endless support to accomplish my work. Special thanks to my family for their love, support and patience that helped me complete my academic epoch. Particular thanks are extended to my colleagues for their support, critical and constructive comments.