The Functional Connectivity of the Human Claustrum According to The
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Imaging Genetic Strategies for Predicting the Quality of Sleep Using Depression-Specific Biomarkers
Imaging genetic strategies for predicting the quality of sleep using depression-specific biomarkers Mansu Kim 1, Xiaohui Yao 1, Bo-yong Park 2, Jingwen Yan 3, and Li Shen1,* 1Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, USA 2McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Canada 3Department of BioHealth Informatics, Indiana University School of Informatics and Computing, Indiana University, USA * Correspondence to [email protected] Overview Joint-connectivity-based sparse CCA Background: Sleep is an essential phenomenon for SNP connectivity Imaging genetics model: The joint-connectivity-based sparse canonical ··· X* samples × maintaining good health and wellbeing [1]. correlation analysis (JCB-SCCA) was applied on preprocessed features n Some studies reported that genetic and p SNPs (Figure 1). JCB-SCCA has an advantage for incorporating connectivity Loading vector u Maximum imaging biomarkers that depression is information and can handle multi-modal neuroimaging datasets. correlation Brain associated with sleep disorder [2]–[4]. connectivity ··· Prior biological knowledge: The average connectivity matrix computed ··· K samples Y* modalitiesn × Imaging genetics: Many studies adopted imaging from the HCP dataset and linkage disequilibrium obtained from 1,000 q voxels genetics methodology to find associations genome project were used as the prior connectivity information of the K = 2 Loading vector V between imaging and genetic biomarkers. In algorithm. The parameters of the algorithm were tuned jointly by nested K = 1 this study, we examine the imaging genetics five-fold cross-validation. Figure 1. JCB-SCCA association in depression and extract biomarkers for predicting the quality of sleep. Experiments and Results Prediction task: We built a prediction model for the Pittsburgh sleep quality index (PSQI) using the identified biomarkers. -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -
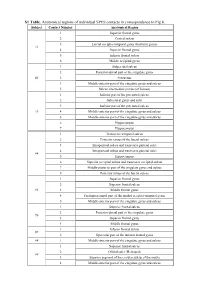
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Amygdaloid Projections to the Ventral Striatum in Mice: Direct and Indirect Chemosensory Inputs to the Brain Reward System
ORIGINAL RESEARCH ARTICLE published: 22 August 2011 NEUROANATOMY doi: 10.3389/fnana.2011.00054 Amygdaloid projections to the ventral striatum in mice: direct and indirect chemosensory inputs to the brain reward system Amparo Novejarque1†, Nicolás Gutiérrez-Castellanos2†, Enrique Lanuza2* and Fernando Martínez-García1* 1 Departament de Biologia Funcional i Antropologia Física, Facultat de Ciències Biològiques, Universitat de València, València, Spain 2 Departament de Biologia Cel•lular, Facultat de Ciències Biològiques, Universitat de València, València, Spain Edited by: Rodents constitute good models for studying the neural basis of sociosexual behavior. Agustín González, Universidad Recent findings in mice have revealed the molecular identity of the some pheromonal Complutense de Madrid, Spain molecules triggering intersexual attraction. However, the neural pathways mediating this Reviewed by: Daniel W. Wesson, Case Western basic sociosexual behavior remain elusive. Since previous work indicates that the dopamin- Reserve University, USA ergic tegmento-striatal pathway is not involved in pheromone reward, the present report James L. Goodson, Indiana explores alternative pathways linking the vomeronasal system with the tegmento-striatal University, USA system (the limbic basal ganglia) by means of tract-tracing experiments studying direct *Correspondence: and indirect projections from the chemosensory amygdala to the ventral striato-pallidum. Enrique Lanuza, Departament de Biologia Cel•lular, Facultat de Amygdaloid projections to the nucleus accumbens, olfactory tubercle, and adjoining struc- Ciències Biològiques, Universitat de tures are studied by analyzing the retrograde transport in the amygdala from dextran València, C/Dr. Moliner, 50 ES-46100 amine and fluorogold injections in the ventral striatum, as well as the anterograde labeling Burjassot, València, Spain. found in the ventral striato-pallidum after dextran amine injections in the amygdala. -

The Connexions of the Amygdala
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.2.137 on 1 April 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 137 The connexions of the amygdala W. M. COWAN, G. RAISMAN, AND T. P. S. POWELL From the Department of Human Anatomy, University of Oxford The amygdaloid nuclei have been the subject of con- to what is known of the efferent connexions of the siderable interest in recent years and have been amygdala. studied with a variety of experimental techniques (cf. Gloor, 1960). From the anatomical point of view MATERIAL AND METHODS attention has been paid mainly to the efferent connexions of these nuclei (Adey and Meyer, 1952; The brains of 26 rats in which a variety of stereotactic or Lammers and Lohman, 1957; Hall, 1960; Nauta, surgical lesions had been placed in the diencephalon and and it is now that there basal forebrain areas were used in this study. Following 1961), generally accepted survival periods of five to seven days the animals were are two main efferent pathways from the amygdala, perfused with 10 % formol-saline and after further the well-known stria terminalis and a more diffuse fixation the brains were either embedded in paraffin wax ventral pathway, a component of the longitudinal or sectioned on a freezing microtome. All the brains were association bundle of the amygdala. It has not cut in the coronal plane, and from each a regularly spaced generally been recognized, however, that in studying series was stained, the paraffin sections according to the Protected by copyright. the efferent connexions of the amygdala it is essential original Nauta and Gygax (1951) technique and the frozen first to exclude a contribution to these pathways sections with the conventional Nauta (1957) method. -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum Along 2 the Dorsal-Ventral Axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.14.876516; this version posted December 15, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Distinct transcriptomic cell types and neural circuits of the subiculum and prosubiculum along 2 the dorsal-ventral axis 3 4 Song-Lin Ding1,2,*, Zizhen Yao1, Karla E. Hirokawa1, Thuc Nghi Nguyen1, Lucas T. Graybuck1, Olivia 5 Fong1, Phillip Bohn1, Kiet Ngo1, Kimberly A. Smith1, Christof Koch1, John W. Phillips1, Ed S. Lein1, 6 Julie A. Harris1, Bosiljka Tasic1, Hongkui Zeng1 7 8 1Allen Institute for Brain Science, Seattle, WA 98109, USA 9 10 2Lead Contact 11 12 *Correspondence: [email protected] (SLD) 13 14 15 Highlights 16 17 1. 27 transcriptomic cell types identified in and spatially registered to “subicular” regions. 18 2. Anatomic borders of “subicular” regions reliably determined along dorsal-ventral axis. 19 3. Distinct cell types and circuits of full-length subiculum (Sub) and prosubiculum (PS). 20 4. Brain-wide cell-type specific projections of Sub and PS revealed with specific Cre-lines. 21 22 23 In Brief 24 25 Ding et al. show that mouse subiculum and prosubiculum are two distinct regions with differential 26 transcriptomic cell types, subtypes, neural circuits and functional correlation. The former has obvious 27 topographic projections to its main targets while the latter exhibits widespread projections to many 28 subcortical regions associated with reward, emotion, stress and motivation. -

Supranuclear and Internuclear Ocular Motility Disorders
CHAPTER 19 Supranuclear and Internuclear Ocular Motility Disorders David S. Zee and David Newman-Toker OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN OCULAR MOTOR SYNDROMES CAUSED BY LESIONS OF THE MEDULLA THE SUPERIOR COLLICULUS Wallenberg’s Syndrome (Lateral Medullary Infarction) OCULAR MOTOR SYNDROMES CAUSED BY LESIONS OF Syndrome of the Anterior Inferior Cerebellar Artery THE THALAMUS Skew Deviation and the Ocular Tilt Reaction OCULAR MOTOR ABNORMALITIES AND DISEASES OF THE OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN BASAL GANGLIA THE CEREBELLUM Parkinson’s Disease Location of Lesions and Their Manifestations Huntington’s Disease Etiologies Other Diseases of Basal Ganglia OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN THE PONS THE CEREBRAL HEMISPHERES Lesions of the Internuclear System: Internuclear Acute Lesions Ophthalmoplegia Persistent Deficits Caused by Large Unilateral Lesions Lesions of the Abducens Nucleus Focal Lesions Lesions of the Paramedian Pontine Reticular Formation Ocular Motor Apraxia Combined Unilateral Conjugate Gaze Palsy and Internuclear Abnormal Eye Movements and Dementia Ophthalmoplegia (One-and-a-Half Syndrome) Ocular Motor Manifestations of Seizures Slow Saccades from Pontine Lesions Eye Movements in Stupor and Coma Saccadic Oscillations from Pontine Lesions OCULAR MOTOR DYSFUNCTION AND MULTIPLE OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN SCLEROSIS THE MESENCEPHALON OCULAR MOTOR MANIFESTATIONS OF SOME METABOLIC Sites and Manifestations of Lesions DISORDERS Neurologic Disorders that Primarily Affect the Mesencephalon EFFECTS OF DRUGS ON EYE MOVEMENTS In this chapter, we survey clinicopathologic correlations proach, although we also discuss certain metabolic, infec- for supranuclear ocular motor disorders. The presentation tious, degenerative, and inflammatory diseases in which su- follows the schema of the 1999 text by Leigh and Zee (1), pranuclear and internuclear disorders of eye movements are and the material in this chapter is intended to complement prominent. -

Revista Brasileira De Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012
Rev Bras Psiquiatr. 2012;34:101-111 Revista Brasileira de Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012 REVIEW ARTICLE Neuroimaging in specific phobia disorder: a systematic review of the literature Ila M.P. Linares,1 Clarissa Trzesniak,1 Marcos Hortes N. Chagas,1 Jaime E. C. Hallak,1 Antonio E. Nardi,2 José Alexandre S. Crippa1 ¹ Department of Neuroscience and Behavior of the Ribeirão Preto Medical School, Universidade de São Paulo (FMRP-USP). INCT Translational Medicine (CNPq). São Paulo, Brazil 2 Panic & Respiration Laboratory. Institute of Psychiatry, Universidade Federal do Rio de Janeiro (UFRJ). INCT Translational Medicine (CNPq). Rio de Janeiro, Brazil Received on August 03, 2011; accepted on October 12, 2011 DESCRIPTORS Abstract Neuroimaging; Objective: Specific phobia (SP) is characterized by irrational fear associated with avoidance of Specific Phobia; specific stimuli. In recent years, neuroimaging techniques have been used in an attempt to better Review; understand the neurobiology of anxiety disorders. The objective of this study was to perform a Anxiety Disorder; systematic review of articles that used neuroimaging techniques to study SP. Method: A literature Phobia. search was conducted through electronic databases, using the keywords: imaging, neuroimaging, PET, spectroscopy, functional magnetic resonance, structural magnetic resonance, SPECT, MRI, DTI, and tractography, combined with simple phobia and specific phobia. One-hundred fifteen articles were found, of which 38 were selected for the present review. From these, 24 used fMRI, 11 used PET, 1 used SPECT, 2 used structural MRI, and none used spectroscopy. Result: The search showed that studies in this area were published recently and that the neuroanatomic substrate of SP has not yet been consolidated. -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69,