Phylogenetic Signal of Threatening Processes Among Hylids: the Need for Clade-Level Conservation Planning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Other Contributions
Other Contributions NATURE NOTES Amphibia: Caudata Ambystoma ordinarium. Predation by a Black-necked Gartersnake (Thamnophis cyrtopsis). The Michoacán Stream Salamander (Ambystoma ordinarium) is a facultatively paedomorphic ambystomatid species. Paedomorphic adults and larvae are found in montane streams, while metamorphic adults are terrestrial, remaining near natal streams (Ruiz-Martínez et al., 2014). Streams inhabited by this species are immersed in pine, pine-oak, and fir for- ests in the central part of the Trans-Mexican Volcanic Belt (Luna-Vega et al., 2007). All known localities where A. ordinarium has been recorded are situated between the vicinity of Lake Patzcuaro in the north-central portion of the state of Michoacan and Tianguistenco in the western part of the state of México (Ruiz-Martínez et al., 2014). This species is considered Endangered by the IUCN (IUCN, 2015), is protected by the government of Mexico, under the category Pr (special protection) (AmphibiaWeb; accessed 1April 2016), and Wilson et al. (2013) scored it at the upper end of the medium vulnerability level. Data available on the life history and biology of A. ordinarium is restricted to the species description (Taylor, 1940), distribution (Shaffer, 1984; Anderson and Worthington, 1971), diet composition (Alvarado-Díaz et al., 2002), phylogeny (Weisrock et al., 2006) and the effect of habitat quality on diet diversity (Ruiz-Martínez et al., 2014). We did not find predation records on this species in the literature, and in this note we present information on a predation attack on an adult neotenic A. ordinarium by a Thamnophis cyrtopsis. On 13 July 2010 at 1300 h, while conducting an ecological study of A. -

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Species Concepts Should Not Conflict with Evolutionary History, but Often Do
ARTICLE IN PRESS Stud. Hist. Phil. Biol. & Biomed. Sci. xxx (2008) xxx–xxx Contents lists available at ScienceDirect Stud. Hist. Phil. Biol. & Biomed. Sci. journal homepage: www.elsevier.com/locate/shpsc Species concepts should not conflict with evolutionary history, but often do Joel D. Velasco Department of Philosophy, University of Wisconsin-Madison, 5185 White Hall, 600 North Park St., Madison, WI 53719, USA Department of Philosophy, Building 90, Stanford University, Stanford, CA 94305, USA article info abstract Keywords: Many phylogenetic systematists have criticized the Biological Species Concept (BSC) because it distorts Biological Species Concept evolutionary history. While defences against this particular criticism have been attempted, I argue that Phylogenetic Species Concept these responses are unsuccessful. In addition, I argue that the source of this problem leads to previously Phylogenetic Trees unappreciated, and deeper, fatal objections. These objections to the BSC also straightforwardly apply to Taxonomy other species concepts that are not defined by genealogical history. What is missing from many previous discussions is the fact that the Tree of Life, which represents phylogenetic history, is independent of our choice of species concept. Some species concepts are consistent with species having unique positions on the Tree while others, including the BSC, are not. Since representing history is of primary importance in evolutionary biology, these problems lead to the conclusion that the BSC, along with many other species concepts, are unacceptable. If species are to be taxa used in phylogenetic inferences, we need a history- based species concept. Ó 2008 Elsevier Ltd. All rights reserved. When citing this paper, please use the full journal title Studies in History and Philosophy of Biological and Biomedical Sciences 1. -

Herramienta Para Evaluar Las Necesidades De Conservación De Anfibio, Panamá, Octubre 2008 Especie En El Rol De Investigación
Herramienta para Evaluar las Necesidades de Conservación de Anfibio, Panamá, Octubre 2008 Page 1 Especie en el Rol de Investigación In Situ 116 especies Una especie que requiere de mayor investigación in situ como parte de las acciones de conservación de esta. Aún falta por conocer al menos una pieza crítica de información. Hábitat Recuperación Estudio Especies Riesgo de extinción Mitigación de amenazas Sobrecolecta protegido de la población filogenético Atelopus varius En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Atelopus zeteki En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Atelopus chiriquiensis En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Oedipina maritima En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Dendrobates arboreus En peligro (EN) Las amenazas no son reversibles ni serán revertidas a tiempo Dendrobates speciosus En peligro (EN) Las amenazas no son reversibles ni serán revertidas a tiempo Craugastor punctariolus En peligro (EN) Las amenazas no son reversibles ni serán revertidas a tiempo Atelopus glyphus En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Bufo fastidiosus En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Bufo peripatetes En peligro crítico (CR) Las amenazas no son reversibles ni serán revertidas a tiempo Herramienta para Evaluar las Necesidades de Conservación de Anfibio, Panamá, Octubre -

Universidade Vila Velha Programa De Pós-Graduação Em Ecologia De Ecossistemas
UNIVERSIDADE VILA VELHA PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA DE ECOSSISTEMAS MODELOS DE NICHO ECOLÓGICO E A DISTRIBUIÇÃO DE PHYLLODYTES (ANURA, HYLIDAE): UMA PERSPECTIVA TEMPORAL DE UM GÊNERO POTENCIALMENTE AMEAÇADO DE EXTINÇÃO POR MUDANÇAS CLIMÁTICAS E INTERAÇÕES BIOLÓGICAS MARCIO MAGESKI MARQUES VILA VELHA FEVEREIRO / 2018 UNIVERSIDADE VILA VELHA PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA DE ECOSSISTEMAS MODELOS DE NICHO ECOLÓGICO E A DISTRIBUIÇÃO DE PHYLLODYTES (ANURA, HYLIDAE): UMA PERSPECTIVA TEMPORAL DE UM GÊNERO POTENCIALMENTE AMEAÇADO DE EXTINÇÃO POR MUDANÇAS CLIMÁTICAS E INTERAÇÕES BIOLÓGICAS Tese apresentada a Universidade Vila Velha, como pré-requisito do Programa de Pós- Graduação em Ecologia de Ecossistemas, para obtenção do título de Doutor em Ecologia. MARCIO MAGESKI MARQUES VILA VELHA FEVEREIRO / 2018 À minha esposa Mariana e meu filho Ângelo pelo apoio incondicional em todos os momentos, principalmente nos de incerteza, muito comuns para quem tenta trilhar novos caminhos. AGRADECIMENTOS Seria impossível cumprir essa etapa tão importante sem a presença do divino Espírito Santo de Deus, de Maria Santíssima dos Anjos e Santos. Obrigado por me fortalecerem, me levantarem e me animarem diante das dificultades, que foram muitas durante esses quatro anos. Agora, servirei a meu Deus em mais uma nova missão. Muito Obrigado. À minha amada esposa Mariana que me compreendeu e sempre esteve comigo me apoiando durante esses quatro anos (na verdade seis, se contar com o mestrado) em momentos de felicidades, tristezas, ansiedade, nervosismo, etc... Esse período nos serviu para demonstrar o quanto é forte nosso abençoado amor. Sem você isso não seria real. Te amo e muito obrigado. Ao meu amado filho, Ângelo Miguel, que sempre me recebia com um iluminado sorriso e um beijinho a cada vez que eu chegava em casa depois de um dia de trabalho. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Filling the Distribution Gap of Boana Exastis (Anura: Hylidae) Within Bahia State, with an Updated Geographic Distribution Map
Herpetology Notes, volume 13: 773-775 (2020) (published online on 24 September 2020) Filling the distribution gap of Boana exastis (Anura: Hylidae) within Bahia State, with an updated geographic distribution map Arielson dos Santos Protázio1,* and Airan dos Santos Protázio2 Boana exastis (Caramaschi and Rodrigues, 2003) is et al., 2018, 2019) mountain ranges in the southwest a stream-breeding tree frog (snout-vent length ca. 88 area of the region known as “Recôncavo Baiano”. The mm) described from southeastern Bahia State, Brazil, second group occurs north of the São Francisco River, and endemic to the Atlantic Forest biome (Caramaschi in fragments of Atlantic Forest in Alagoas State, in and Rodrigues, 2003; Loebmann et al., 2008). Its dorsal the municipalities of Quebrangulo (Silva et al., 2008), colour pattern (similar to lichen) and the presence of Ibateguara (Bourgeois, 2010), Boca da Mata (Palmeira crenulated fringes on the arms and legs led Caramaschi and Gonçalvez, 2015), Maceió, Murici and Passo do and Rodrigues (2003) to determine that B. exastis Camaragibe (Almeida et al., 2016), and in Pernambuco belonged to the Boana boans group, and revealed that it State, in the municipalities of Jaqueira (Private Reserve was closely related to B. lundii (Burmeister, 1856) and of Natural Heritage - RPPN Frei Caneca; Santos and B. pardalis (Spix, 1824). Later, B. exastis was included Santos, 2010) and Lagoa dos Gatos (RPPN Pedra within the Boana faber group (Faivovich et al., 2005). D’anta; Roberto et al., 2017). Comparisons between their acoustic features and calling This information reveals a gap regarding the sites indicated that B. exastis is more closely related to occurrence of B. -
For Review Only
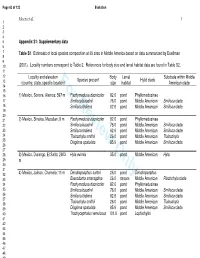
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Pseudoeurycea Naucampatepetl. the Cofre De Perote Salamander Is Endemic to the Sierra Madre Oriental of Eastern Mexico. This
Pseudoeurycea naucampatepetl. The Cofre de Perote salamander is endemic to the Sierra Madre Oriental of eastern Mexico. This relatively large salamander (reported to attain a total length of 150 mm) is recorded only from, “a narrow ridge extending east from Cofre de Perote and terminating [on] a small peak (Cerro Volcancillo) at the type locality,” in central Veracruz, at elevations from 2,500 to 3,000 m (Amphibian Species of the World website). Pseudoeurycea naucampatepetl has been assigned to the P. bellii complex of the P. bellii group (Raffaëlli 2007) and is considered most closely related to P. gigantea, a species endemic to the La specimens and has not been seen for 20 years, despite thorough surveys in 2003 and 2004 (EDGE; www.edgeofexistence.org), and thus it might be extinct. The habitat at the type locality (pine-oak forest with abundant bunch grass) lies within Lower Montane Wet Forest (Wilson and Johnson 2010; IUCN Red List website [accessed 21 April 2013]). The known specimens were “found beneath the surface of roadside banks” (www.edgeofexistence.org) along the road to Las Lajas Microwave Station, 15 kilometers (by road) south of Highway 140 from Las Vigas, Veracruz (Amphibian Species of the World website). This species is terrestrial and presumed to reproduce by direct development. Pseudoeurycea naucampatepetl is placed as number 89 in the top 100 Evolutionarily Distinct and Globally Endangered amphib- ians (EDGE; www.edgeofexistence.org). We calculated this animal’s EVS as 17, which is in the middle of the high vulnerability category (see text for explanation), and its IUCN status has been assessed as Critically Endangered. -

Phylogeography of the American Green Treefrog Species Group
PHYLOGEOGRAPHY OF THE AMERICAN GREEN TREEFROG SPECIES GROUP by PAUL NATHANIEL PASICHNYK Presented to the Faculty of the Graduate School of The University of Texas at Arlington In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON November 2016 Copyright © by Paul N. Pasichnyk 2016 All Rights Reserved ii Acknowledgments A student’s graduate work is definitely not something that is done alone. I have received help from numerous people over the years and without their contributions, this would not have been possible. First, I would like to thank my parents for their tremendous support and motivation as I grew up and provided me with a foundation and love in education. Secondly, I would like to thank the person who has had to help me daily with tasks as simple as a pat on the back to as complex as reading a rough draft of a dissertation that might as well be in a foreign language, my wife Karen. My in-laws deserve special thanks as well, I do not believe that it is possible for others’ in-laws to be as supportive and helpful as mine. Then I would like to thank all the undergrads and friends who have helped see this to completion: Richard Hanna, Sari Mahon, Sarah Young, Nicole Lopez, Annamarie Slaven, Leslie Segovia, Pankaj BC, Matt Moseley, Christian Cox, Jeff Streicher, Claudia Marquez, Jesse Meik, Walter Schargel, Corey Roelke, Matt Watson, Rebbekah Watson, Thomas Eimermacher, Utpal Smart, David Sanchez, Jacobo Reyes-Velasco, Melissa Muenzler, Carl Franklin, Linda Taylor, Gloria Burlingham, Sherri Echols, and Paulette Williams. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species.