JBES-Vol-12-No-4-P-1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Final Report
Final Report Baseline Study for Hand in Hand Eastern Africa Program in Northern Tanzania August 2017 Table of Contents Table of Contents.................................................................................................................. 2 List of tables.......................................................................................................................... 3 List of figures ........................................................................................................................ 4 List of Abbreviations.............................................................................................................. 5 Acknowledgement................................................................................................................. 6 Executive Summary .............................................................................................................. 7 1. Introduction.................................................................................................................. 13 1.1 Overview of HiH EA Model.................................................................................... 13 1.2 HiH EA Expansion Plan for Tanzania.................................................................... 14 2. The Baseline Study...................................................................................................... 15 2.1 The Scoping Study................................................................................................ 15 2.1.1 Objectives of the Scoping -

Arusha District Council
ARUSHA DISTRICT COUNCIL INVESTMENT PROFILE DISCLOSE THE POTENTIAL OF ARUSHA DISTRICT COUNCIL February, 2017 FOREWORD I would like to welcome all esteemed investors to explore the irresistible opportunities provided by the Arusha District Council. Arusha District Council was established in 2007, the Council has a vision of to be a leading transformed Council that provides high quality services for Sustainable Development of the Community by 2025. In order to increase competitiveness in attracting investors to our District Council, effort and initiative to identify, expose and promote investment opportunities available in Arusha District Council is going on. We are indeed determined to utilize potential areas owned by the Council, Communities and those own by private developer. In this Investment Profile, we give you opportunities to realize your entrepreneurial ambitions and explore them. We believe in supporting our investors’ aspirations as the Council. As we want to be one among the leading investment avenues in Tanzania. In Arusha District Council investors are favoured with presence of suitable investment climate that will help you capitalize on untapped opportunities in Arusha and Tanzania as a whole. Whereby investment can be done to the following areas of interests include tourism, processing industries, livestock and agricultural sector, beekeeping, sports and recreation centre, modern market, real estate, socio-economic services. Investment climate is characterised by peace and stability, availability of raw materials, market, abundant natural resources, road and transportation network, electricity services and the strategic geographical location will support establishment and success of investments. It is because of the above mention few facts we are proud to say that Arusha District Council is the best investment destination of your choice. -

Progress Report: GMH (#0090): an Integrated Approach to Addressing the Issue of Adolescent Depression In
Progress Report: GMH (#0090): An Integrated Approach to Addressing the Issue of Adolescent Depression in Malawi and Tanzania August 5, 2014 Submitted To: Grand Challenges Canada Submitted By: Farm Radio International Progress against milestones for period ending July 15, 2014 MoUs signed with Ministry Health and Education in Tanzania To date, we have held two stakeholder meetings and one mental health training to secure buy-in from relevant government ministries. Representatives from the regional and district level MoE and MoH were invited to attend the meetings. We have also had one face-to-face meeting with the Director of Mental Health Services in the Ministry of Health in Dar es Salam. Representatives from the ministries have indicated their enthusiasm and support for the project, and their willingness to form a technical advisory group and to sing Memoranda of Understanding for the project. MoUs have been sent to the national MoE and MoH, and are awaiting a reply 35 schools selected for participation in target districts in Tanzania 35 secondary schools have been selected for participation in the program, and we have received clearance to conduct activities by the regional and district level representatives from the Ministry of Education. To date, we have completed a baseline survey of 350 youth from 10 schools, and will survey another 350 youth from an additional 10 schools by the end of August, 2014. Attached (annex 1) is a list of 35 schools selected for participation in the program 2 MOUs signed with radio stations We have selected 2 radio stations in the Arusha region to participate in the communication component of the program in Tanzania. -

Tanzania Institute of Accountancy-Kigoma Campus
TANZANIA INSTITUTE OF ACCOUNTANCY-KIGOMA CAMPUS NO F4 INDEX NO SEX NAME SECONDARY SCHOOL PROGRAM SELECTED 1 S0136/0015 M CHARLES S RAPHAEL MUSOMA SECONDARY SCHOOL BTCBA 2 S0178/0048 M RASHID ABRAHAMU ERNEST MANOW LUTHERAN JUNIOR SEMINARY BTCBA 3 S0181/0033 F MONICA ROGERS KISARAWE LUTHERAN JUNIOR SEMINARY BTCBA 4 S0266/0067 F VAILETH H BALAGAJU REGINA MUNDI GIRLS SECONDARY SCHOOL BTCBA 5 S0274/0027 F JUDITH J KILAVE SUNSHINE SECONDARY SCHOOL BTCBA 6 S0307/0095 M YOHANA MABENANGA PAULO DODOMA CENTRAL SECONDARY SCHOOL BTCBA 7 S0308/0196 M SISTY MICHAEL MASSAWE ENABOISHU SECONDARY SCHOOL BTCBA 8 S0359/0073 F NASMA H ALFANI KIGURUNYEMBE SECONDARY SCHOOL BTCBA 9 S0361/0089 F THERESIA DAMIAN KAWONGA SINGE SECONDARY SCHOOL BTCBA 10 S0362/0065 M HAMIS ABRAHAMAN SAID IHANJA SECONDARY SCHOOL BTCBA 11 S0372/0071 M JULIAS ASTERI MUSHI KIRUA SECONDARY SCHOOL BTCBA 12 S0374/0001 F ABIGAEL MWAKANDYALI LUPATA SECONDARY SCHOOL BTCBA 13 S0378/0003 F CATHERINE STEVEN MHINA HEGONGO HOLY CROSS SECONDARY SCHOOL BTCBA 14 S0419/0057 M MICHAEL DAVID LUHAMBA CHOME SECONDARY SCHOOL BTCBA 15 S0429/0035 M BEATUS YONAH NGENIUKO LUPEMBE SECONDARY SCHOOL BTCBA 16 S0429/0057 M HUMPHREY JOSEPH LIHAWA LUPEMBE SECONDARY SCHOOL BTCBA 17 S0445/0001 F AMINA SEIF MTAUKA MWEMBETOGWA SECONDARY SCHOOL BTCBA 18 S0456/0244 M SELEMANI FADHIL MARIJANI KINANGO SECONDARY SCHOOL BTCBA 19 S0462/0022 M COSMAS JACOB JOHN WIGEHE SECONDARY SCHOOL BTCBA 20 S0473/0123 M ISMAIL ABDALLAH KANYIGO SECONDARY SCHOOL BTCBA 21 S0476/0102 M AWE MATHEW MWAMBUSISYE MWANGAZA SECONDARY SCHOOL BTCBA 22 S0499/0072 -

Parthenium Hysterophorus L.) ON
i IMPACT OF PARTHENIUM WEED (Parthenium hysterophorus L.) ON PRODUCTION OF MAIZE AND COMMON BEANS IN ARUSHA, TANZANIA HAMIS DANIEL WAMBURA A DISSERTATION SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CROP SCIENCE OF SOKOINE UNIVERSITY OF AGRICULTURE, MOROGORO, TANZANIA 2018 i EXTENDED ABSTRACT Introduction and spread of Parthenium hysterophorus is affecting crop production and farmer’s health. Impacts of this weed on crop production need be assessed and effective management measures be developed in order to reduce or prevent its effects. A study was therefore carried out in Arusha and Meru districts, to (i) describe the farmer’s perceptions about parthenium weed (ii) establish distribution and abundance of parthenium weed (iii) assess effective methods for parthenium weed control in maize fields. To achieve the above mentioned objectives, a field survey was conducted during the long rain season of 2017 in the study areas where 120 farmers growing maize and common beans were interviewed. List of villages and farmers were obtained from the district agricultural officers in the two districts. Three villages from Meru district (Mbuguni, Nasholi and Mareu) were selected randomly while Ilkerini, Losikito and Olorieni were randomly selected from Arusha district. Selection of 20 farmers from each village was also done randomly. On distribution and abundance of the weed, latitudes, longitudes and altitudes were recorded for every 10km intervals along the main arterial roads leading out of Arusha to Namanga, Kilimanjaro Airport, Mbuguni and Ilkerini using a hand-held GPS and simultaneously absence or presence and abundance were recorded. In order to identify effective methods to control the parthenium weed, a field experiment was carried out in a randomized complete block design (CRBD) with four replications at the Tropical Pesticides Research Institute (TPRI) in Arusha. -

Northern Zone Regions Investment Opportunities
THE UNITED REPUBLIC OF TANZANIA PRIME MINISTER’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT Arusha “The centre for Tourism & Cultural heritage” NORTHERN ZONE REGIONS INVESTMENT OPPORTUNITIES Kilimanjaro “Home of the snow capped mountain” Manyara “Home of Tanzanite” Tanga “The land of Sisal” NORTHERN ZONE DISTRICTS MAP | P a g e i ACRONYMY AWF African Wildlife Foundation CBOs Community Based Organizations CCM Chama cha Mapinduzi DC District Council EPZ Export Processing Zone EPZA Export Processing Zone Authority GDP Gross Domestic Product IT Information Technology KTC Korogwe Town Council KUC Kilimanjaro Uchumi Company MKUKUTA Mkakati wa Kukuza Uchumi na Kupunguza Umaskini Tanzania NDC National Development Corporation NGOs Non Government Organizations NSGPR National Strategy for Growth and Poverty Reduction NSSF National Social Security Fund PANGADECO Pangani Development Corporation PPP Public Private Partnership TaCRI Tanzania Coffee Research Institute TAFIRI Tanzania Fisheries Research Institute TANROADS Tanzania National Roads Agency TAWIRI Tanzania Wildlife Research Institute WWf World Wildlife Fund | P a g e ii TABLE OF CONTENTS ACRONYMY ............................................................................................................ii TABLE OF CONTENTS ........................................................................................... iii 1.0 INTRODUCTION ..............................................................................................1 1.1 Food and cash crops............................................................................................1 -

ICS Market Intelligence Arusha & Manyara
Market Intelligence on Improved Cook Stoves in Manyara and Arusha Regions ICS Taskforce Tanzania October 2013 About ICS Taskforce Facilitated by SNV, the ICS Taskforce of Tanzania was created in 2011, with the Ministry of Energy and Minerals (MEM) as the Chair and the Tanzania Renewable Energy Association (TAREA) elected as the secretariat. The ICS Taskforce was initiated with the aim to increase coordination in the Improved Cook Stove (ICS) sector, for stakeholders to better understand and develop the sector through multi-stakeholder processes, while doing the necessary studies to come to a joint way forward for further ICS market development in the country. This document is one of the resulting documents of the ICS Taskforce. Other documents include: a technical assessment report of ICS in Tanzania, market intelligence studies for ICS in different regions of the country, ICS policy analysis, and a Country Action Plan for Clean Cookstoves and Fuels. Authors: Livinus Manyanga, Goodluck Makundi, Lucy Morewa, Jacqueline Mushi – KAKUTE Coordination and editing: Finias Magessa & Martijn Veen, SNV Tanzania Photos cover page: Josh Sebastian (middle & right) and Mzumbe Musa (left) ISBN: 978-9987-9895-3-9 Disclaimer Any views or opinions presented in this publication are solely those of the authors and do not necessarily represent those of SNV, TAREA, or any other institutional member of the ICS Taskforce, and should not be directly attributed to any of the individuals interviewed or organizations involved unless quoted verbatim. Whilst the utmost care has been made in compiling accurate information for this report, SNV cannot guarantee it is factual accurate or up to date at the time of reading. -

Download Document
GS-VPA-VCR-FORM Verification and certification report form for GS Voluntary Project Activity Complete this form in accordance with the instructions attached at the end of this form. BASIC INFORMATION Title and GS reference number of the African Biogas Carbon Programme (ABC) – Tanzania – Voluntary Project Activity (VPA) CAMARTEC - VPA002 GS: 2751 (PoA: African Biogas Carbon Programme (ABC) (GS2747)) Project No: 19/037 – MY-PVerGS 19/05 Version number(s) of the VPA-DD to which this report applies 10.0 Version number of the verification and certification report 1.3 Completion date of the verification and certification report 17/10/2019 Monitoring period number and duration MP: 2 of this morning period VPA002: Duration: 01/01/2015 – 31/12/2018 both dates inclusive Number and version number of the Number: 1 monitoring report to which this report applies Version: 1.6 Coordinating/managing entity (CME) HIVOS Foundation Host Parties Is this a host Party to a CPA Host Parties of the PoA covered in this report? (yes/no) Tanzania No Applied methodologies and standardized Technologies and Practices to Displace Decentralized baselines Thermal Energy Consumption (version 2.0) Mandatory sectoral scopes linked to the Scope 1: Energy industries (renewable-/non-renewable applied methodologies sources) Scope 13: Waste handling and disposal1 Conditional sectoral scopes linked to the applied methodologies, if applicable N/A Estimated amount of GHG emission reductions or GHG removals for this 463,196 tCO2e monitoring period in the included VPAs 1 VPA-DD refers to Scope 15 Agriculture whereas the related GS webpage https://www.goldstandard.org/resources/energy-requirements 1, 3 and 13 for this methodology. -

The Status of Meru Language: Domain of Use, Intergeneration Transmission and Speakers’ Attitude
The University of Dodoma University of Dodoma Institutional Repository http://repository.udom.ac.tz Social Sciences Master Dissertations 2017 The status of Meru language: domain of use, intergeneration transmission and þÿspeakers attitude Pallangyo, Wariaeli Ismaeli The University of Dodoma Pallangyo, W. I. (2017). The status of Meru language: domain of use, intergeneration þÿtransmission and speakers attitude. Dodoma: The University of Dodoma http://hdl.handle.net/20.500.12661/431 Downloaded from UDOM Institutional Repository at The University of Dodoma, an open access institutional repository. THE STATUS OF MERU LANGUAGE: DOMAIN OF USE, INTERGENERATION TRANSMISSION AND SPEAKERS’ ATTITUDE WARIAELI ISMAELI PALLANGYO MASTER OF ARTS IN LINGUISTICS THE UNIVERSITY OF DODOMA OCTOBER, 2017 THE STATUS OF MERU LANGUAGE: DOMAIN OF USE, INTERGENERATION TRANSMISSION AND SPEAKERS’ ATTITUDE By Wariaeli Ismaeli Pallangyo A Dissertation submitted in partial fulfillment of the requirements for the degree of Master of Arts in Linguistics of the University of Dodoma The University of Dodoma October, 2017 CERTIFICATION The undersigned certifies that she has read and hereby recommends for acceptance by the University of Dodoma, a dissertation entitled the Status of Meru Language in Tanzania: Domain of Use, Intergeneration Transmission and Speakers’ Attitude, in partial fulfillment of the requirements for the Degree of Master of Arts in Linguistics of the University of Dodoma. …………………………… (Supervisor) Dr. Chrispina Alphonce Date............................... i DECLARATION AND COPYRIGHT I, Wariaeli I. Pallangyo, declare that this dissertation is my own original work and that it has not been presented and will not be presented to any other University for a similar or any other degree award. Signature............................................. -

Farmer Testimonials Chicken Vaccination N Tanzania.Pdf
Farmers in RIU networks in N. Tanzania benefit from increasing chicken populations Promotion Update: April 2011 Background: There are numerous constraints to increased indigenous chicken production by small- holder farmers in Tanzania. These include disease, predation, and lack of information on improved husbandry. Most farmers perceive chickens to have little value and leave them to freely range to scavenge for food. However, in the process, young chicks often fall prey to avian predators such as the hawk which may take up to 100% of young chicks. Any chicks that survive the predators often succumb to the Newcastle disease. Because farmers typically have a small number of chickens (less than 10), vaccination against the disease is not economic because vaccines are packed in doses of 400 for the larger commercial farmers. As a result, chickens Image: Aliapenda, a FIPS-Africa VBA has been saving are mostly kept as a hobby rather than a business. money from chicken vaccination to help her start a business. With the support of DFID’s Research Into Use programme (RIU), FIPS-Africa has established networks of self-employed Village-based Advisors (VBAs) in Moshi and Meru districts, trained by Ministry of Agriculture staff, with the aim of helping small-holder farmers gain access to the appropriate farm inputs, and information on their best management. In the process, VBAs generate income from a number of activities such as the sale of improved seeds and fertilizers. This income helps to sustain their activities They also offer services such as vaccination of indigenous chickens, and dyeing of chicks with Gentian Violet (GV) to prevent Image: A FIPS-Africa VBA demonstrating how to dye a chick predation by birds of prey. -

Fund Shall Maintain a Register of Universal Service Provision Which Shall Include the Following
UNIVERSAL COMMUNICATIONS SERVICE ACCESS FUND PUBLIC REGISTER OF UNIVERSAL SERVICE PROVISION 1. INTRODUCTION The Government of the United Republic of Tanzania established the Universal Communications Service Access Fund (the Fund) to promote universal access to information and communications technology services in Tanzania. The Fund is responsible for enabling accessibility and participation by communication operators in the provision of communication services, with a view to promoting social, economic development of the rural and urban underserved areas; to provide for availability of communication services by establishing a legal framework for universal service providers to meet the communication needs of consumers. Section 15 of The Universal Communications Service Access Act, 2006 provides designation of universal service providers. Regulation 28(1) of the UCSAF’s Regulations of 2009 states that The Fund shall maintain a register of universal service provision which shall include the following:- a) Designated service providers; b) A list of licensees contributing to the Fund; and c) A list of information required by the Fund when determining designated universal service providers. Following this requirement, the Fund has developed this register that is subject to update from time to time. 1 2. DESIGNATED SERVICE PROVIDERS The following section presents designated service providers for universal service provision. These are mainly mobile operator service providers whose main responsibility is to extend mobile communication in rural and urban underserved areas of the country. Through the subsidy provided by the Fund, currently 992 wards will receive communication service through these designated service providers as indicated in Annex 1 of this register. In addition, other projects including school connectivity are included starting from Annex 1A to annex 1I. -
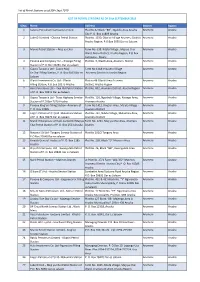
List of Petrol Stations As of 30Th Sept 2019 S/No. Name Address District
List of Petrol Stations as of 30th Sept 2019 LIST OF PETROL STATIONS AS OF 30th SEPTEMBER 2019 S/No. Name Address District Region 1 Saiteru Petroleum Company Limited Plot No.32 Block “BB”, Ngulelo Area Arusha Arumeru Arusha City P. O. Box 11809 Arusha 2 Lake Oil Limited - Olosiva Petrol Station Plot No. 1330, Olosiva Village Arumeru District Arumeru Arusha Arusha Region, P.O.Box 5055 Dar es Salaam 3 Munio Petrol Station – Maji ya Chai Farm No. 039, Kitefu Village , Maji ya Chai Arumeru Arusha Ward, Meru District, Arusha Region, P.O Box 160 Himo - Moshi 4 Panone and Company Ltd – Kisongo Filling Plot No. 3, Olasiti Area, Arumeru District Arumeru Arusha Station of P.O. Box 33285, Dar es salaam 5 Gapco Tanzania Ltd - Gapco Maji Farm No 1118 Imbaseni Village Arumeru Arusha Ya Chai Filling Station, P. O. Box 9103 Dar es Arumeru District in Arusha Region Salaam 6 Olasiti Investment Co. Ltd - Olasiti Plot no 46 Olasiti Area Arumeru Arumeru Arusha Filling Station, P.O.Box 10275 Arusha District, Arusha Region 7 Hass Petroleum Ltd – Kwa Idd Petrol Station Plot No. 832, Arumeru District, Arusha Region Arumeru Arusha of P. O. Box 78341 Dar es Salaam 8 Gapco Tanzania Ltd - Trans Highway Service Plot No. 116, Ngorbob Village, Kisongo Area, Arumeru Arusha Station of P.O Box 7370 Arusha Arumeru Arusha 9 Panone King’ori Filling Station- Arumeru of Farm No.1411, King’ori Area, Malula Village - Arumeru Arusha P. O. Box 33285 Arumeru District 10 Engen Petroleum (T) Ltd- Makumira Station Plot No. 797, Ndato Village, Makumira Area, Arumeru Arusha of P.