Parthenium Hysterophorus L.) ON
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Report
Final Report Baseline Study for Hand in Hand Eastern Africa Program in Northern Tanzania August 2017 Table of Contents Table of Contents.................................................................................................................. 2 List of tables.......................................................................................................................... 3 List of figures ........................................................................................................................ 4 List of Abbreviations.............................................................................................................. 5 Acknowledgement................................................................................................................. 6 Executive Summary .............................................................................................................. 7 1. Introduction.................................................................................................................. 13 1.1 Overview of HiH EA Model.................................................................................... 13 1.2 HiH EA Expansion Plan for Tanzania.................................................................... 14 2. The Baseline Study...................................................................................................... 15 2.1 The Scoping Study................................................................................................ 15 2.1.1 Objectives of the Scoping -

Arusha District Council
ARUSHA DISTRICT COUNCIL INVESTMENT PROFILE DISCLOSE THE POTENTIAL OF ARUSHA DISTRICT COUNCIL February, 2017 FOREWORD I would like to welcome all esteemed investors to explore the irresistible opportunities provided by the Arusha District Council. Arusha District Council was established in 2007, the Council has a vision of to be a leading transformed Council that provides high quality services for Sustainable Development of the Community by 2025. In order to increase competitiveness in attracting investors to our District Council, effort and initiative to identify, expose and promote investment opportunities available in Arusha District Council is going on. We are indeed determined to utilize potential areas owned by the Council, Communities and those own by private developer. In this Investment Profile, we give you opportunities to realize your entrepreneurial ambitions and explore them. We believe in supporting our investors’ aspirations as the Council. As we want to be one among the leading investment avenues in Tanzania. In Arusha District Council investors are favoured with presence of suitable investment climate that will help you capitalize on untapped opportunities in Arusha and Tanzania as a whole. Whereby investment can be done to the following areas of interests include tourism, processing industries, livestock and agricultural sector, beekeeping, sports and recreation centre, modern market, real estate, socio-economic services. Investment climate is characterised by peace and stability, availability of raw materials, market, abundant natural resources, road and transportation network, electricity services and the strategic geographical location will support establishment and success of investments. It is because of the above mention few facts we are proud to say that Arusha District Council is the best investment destination of your choice. -

Northern Zone Regions Investment Opportunities
THE UNITED REPUBLIC OF TANZANIA PRIME MINISTER’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT Arusha “The centre for Tourism & Cultural heritage” NORTHERN ZONE REGIONS INVESTMENT OPPORTUNITIES Kilimanjaro “Home of the snow capped mountain” Manyara “Home of Tanzanite” Tanga “The land of Sisal” NORTHERN ZONE DISTRICTS MAP | P a g e i ACRONYMY AWF African Wildlife Foundation CBOs Community Based Organizations CCM Chama cha Mapinduzi DC District Council EPZ Export Processing Zone EPZA Export Processing Zone Authority GDP Gross Domestic Product IT Information Technology KTC Korogwe Town Council KUC Kilimanjaro Uchumi Company MKUKUTA Mkakati wa Kukuza Uchumi na Kupunguza Umaskini Tanzania NDC National Development Corporation NGOs Non Government Organizations NSGPR National Strategy for Growth and Poverty Reduction NSSF National Social Security Fund PANGADECO Pangani Development Corporation PPP Public Private Partnership TaCRI Tanzania Coffee Research Institute TAFIRI Tanzania Fisheries Research Institute TANROADS Tanzania National Roads Agency TAWIRI Tanzania Wildlife Research Institute WWf World Wildlife Fund | P a g e ii TABLE OF CONTENTS ACRONYMY ............................................................................................................ii TABLE OF CONTENTS ........................................................................................... iii 1.0 INTRODUCTION ..............................................................................................1 1.1 Food and cash crops............................................................................................1 -

The Status of Meru Language: Domain of Use, Intergeneration Transmission and Speakers’ Attitude
The University of Dodoma University of Dodoma Institutional Repository http://repository.udom.ac.tz Social Sciences Master Dissertations 2017 The status of Meru language: domain of use, intergeneration transmission and þÿspeakers attitude Pallangyo, Wariaeli Ismaeli The University of Dodoma Pallangyo, W. I. (2017). The status of Meru language: domain of use, intergeneration þÿtransmission and speakers attitude. Dodoma: The University of Dodoma http://hdl.handle.net/20.500.12661/431 Downloaded from UDOM Institutional Repository at The University of Dodoma, an open access institutional repository. THE STATUS OF MERU LANGUAGE: DOMAIN OF USE, INTERGENERATION TRANSMISSION AND SPEAKERS’ ATTITUDE WARIAELI ISMAELI PALLANGYO MASTER OF ARTS IN LINGUISTICS THE UNIVERSITY OF DODOMA OCTOBER, 2017 THE STATUS OF MERU LANGUAGE: DOMAIN OF USE, INTERGENERATION TRANSMISSION AND SPEAKERS’ ATTITUDE By Wariaeli Ismaeli Pallangyo A Dissertation submitted in partial fulfillment of the requirements for the degree of Master of Arts in Linguistics of the University of Dodoma The University of Dodoma October, 2017 CERTIFICATION The undersigned certifies that she has read and hereby recommends for acceptance by the University of Dodoma, a dissertation entitled the Status of Meru Language in Tanzania: Domain of Use, Intergeneration Transmission and Speakers’ Attitude, in partial fulfillment of the requirements for the Degree of Master of Arts in Linguistics of the University of Dodoma. …………………………… (Supervisor) Dr. Chrispina Alphonce Date............................... i DECLARATION AND COPYRIGHT I, Wariaeli I. Pallangyo, declare that this dissertation is my own original work and that it has not been presented and will not be presented to any other University for a similar or any other degree award. Signature............................................. -

Farmer Testimonials Chicken Vaccination N Tanzania.Pdf
Farmers in RIU networks in N. Tanzania benefit from increasing chicken populations Promotion Update: April 2011 Background: There are numerous constraints to increased indigenous chicken production by small- holder farmers in Tanzania. These include disease, predation, and lack of information on improved husbandry. Most farmers perceive chickens to have little value and leave them to freely range to scavenge for food. However, in the process, young chicks often fall prey to avian predators such as the hawk which may take up to 100% of young chicks. Any chicks that survive the predators often succumb to the Newcastle disease. Because farmers typically have a small number of chickens (less than 10), vaccination against the disease is not economic because vaccines are packed in doses of 400 for the larger commercial farmers. As a result, chickens Image: Aliapenda, a FIPS-Africa VBA has been saving are mostly kept as a hobby rather than a business. money from chicken vaccination to help her start a business. With the support of DFID’s Research Into Use programme (RIU), FIPS-Africa has established networks of self-employed Village-based Advisors (VBAs) in Moshi and Meru districts, trained by Ministry of Agriculture staff, with the aim of helping small-holder farmers gain access to the appropriate farm inputs, and information on their best management. In the process, VBAs generate income from a number of activities such as the sale of improved seeds and fertilizers. This income helps to sustain their activities They also offer services such as vaccination of indigenous chickens, and dyeing of chicks with Gentian Violet (GV) to prevent Image: A FIPS-Africa VBA demonstrating how to dye a chick predation by birds of prey. -
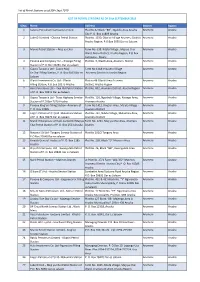
List of Petrol Stations As of 30Th Sept 2019 S/No. Name Address District
List of Petrol Stations as of 30th Sept 2019 LIST OF PETROL STATIONS AS OF 30th SEPTEMBER 2019 S/No. Name Address District Region 1 Saiteru Petroleum Company Limited Plot No.32 Block “BB”, Ngulelo Area Arusha Arumeru Arusha City P. O. Box 11809 Arusha 2 Lake Oil Limited - Olosiva Petrol Station Plot No. 1330, Olosiva Village Arumeru District Arumeru Arusha Arusha Region, P.O.Box 5055 Dar es Salaam 3 Munio Petrol Station – Maji ya Chai Farm No. 039, Kitefu Village , Maji ya Chai Arumeru Arusha Ward, Meru District, Arusha Region, P.O Box 160 Himo - Moshi 4 Panone and Company Ltd – Kisongo Filling Plot No. 3, Olasiti Area, Arumeru District Arumeru Arusha Station of P.O. Box 33285, Dar es salaam 5 Gapco Tanzania Ltd - Gapco Maji Farm No 1118 Imbaseni Village Arumeru Arusha Ya Chai Filling Station, P. O. Box 9103 Dar es Arumeru District in Arusha Region Salaam 6 Olasiti Investment Co. Ltd - Olasiti Plot no 46 Olasiti Area Arumeru Arumeru Arusha Filling Station, P.O.Box 10275 Arusha District, Arusha Region 7 Hass Petroleum Ltd – Kwa Idd Petrol Station Plot No. 832, Arumeru District, Arusha Region Arumeru Arusha of P. O. Box 78341 Dar es Salaam 8 Gapco Tanzania Ltd - Trans Highway Service Plot No. 116, Ngorbob Village, Kisongo Area, Arumeru Arusha Station of P.O Box 7370 Arusha Arumeru Arusha 9 Panone King’ori Filling Station- Arumeru of Farm No.1411, King’ori Area, Malula Village - Arumeru Arusha P. O. Box 33285 Arumeru District 10 Engen Petroleum (T) Ltd- Makumira Station Plot No. 797, Ndato Village, Makumira Area, Arumeru Arusha of P. -

Improving Accessibility of Safe and Clean Water In
IMPROVING ACCESSIBILITY OF SAFE AND CLEAN WATER IN MARORONI WARD IN MERU DISTRICT, ARUSHA NEEMA JOSIAH SAMBO A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT FOR THE REQUIREMENTS FOR THE DEGREE OF MASTERS IN COMMUNITY ECONOMIC DEVELOPMENT OF THE OPEN UNIVERSITY OF TANZANIA 2014 ii CERTIFICATION This is to certify that I have read this project paper and I’m satisfied that it can be submitted to the Senate of the Open University of Tanzania in partial fulfillment of the requirements for the award of Master’s degree in Community Economic Development (M. CED). ……………………………... Dr. Felician Mutasa (Supervisor) ……………………….. Date iii COPYRIGHT No part of this project paper may be reproduced, stored in any retrieval system, or transmitted in any form by any means, electronic, mechanical, photocopying, recording or otherwise without prior written permission of the author or the Open University of Tanzania. iv DECLARATION I, Neema Sambo, do hereby declare that this is my own original work and it has not in part or wholly been presented for a degree of Masters in Community Economic Development or any other degree at any University. ……………………………. Signature ……………………….. Date v DEDICATION To my husband, and our dear children Joshua and Amon, who patiently endured my absence while I was busy with the study. vi ACKNOWLEDGEMENTS I would like to acknowledge the support of family, friends, faculty and institutions, who have stood with me in my efforts to complete this stage of my academic journey. I will certainly not be able to name them individually, by doing so would make me vulnerable to the accusation of not being grateful to some. -

Letter from the Director
VIEW ONLINE / FORWARD / SUBSCRIBE / UNSUBSCRIBE SHARE: Q2 2016 Letter from the Director Our fourth year is off to a busy start! We are now implementing BID solutions in a third district in Tanzania, and in Zambia, we are preparing for launch in Southern Province, currently testing the new electronic immunization registry and fine-tuning other data- use interventions. Last quarter found our team across the world meeting with partners, Melinda Gates, and key stakeholders to continue to ensure BID’s solutions are not only relevant for countries across sub-Saharan Africa, but are sustainable and can scale beyond our test sites and into other health areas. Additionally, we are working with the ministries of health to do resource planning for how our demonstration countries can scale nationally, as well as developing the package of BID solutions that can be adapted to other country contexts. Please continue to follow along our journey by finding us onFacebook and Twitter and subscribing to our blog. Sincerely, Laurie Werner Global Director, BID Initiative PATH Si vous désirez recevoir la version française de notre bulletin d'information, veuillez vous abonner ici. - Melinda Gates Visits the BID Initiative Photo: Bill & Melinda Gates Foundation/Riccardo Gangale. Melinda Gates visits Magomeni Health Facility in Tanzania. We were truly honored to host Melinda Gates in Tanzania this June. As the co-chair of the Bill & Melinda Gates Foundation, our funder, we were pleased to demonstrate our progress to date toward enhancing immunization and overall health service delivery through improved data collection, quality, and use. Melinda Gates joined BID’s Henry Mwanyika and Hassan Mtenga, nurse Paulo Urioh from Mareu Health Facility in Arusha, government officials, and facility staff at Magomeni Health Facility in Dar es Salaam, Tanzania. -

Appendices to Vol 4B
Vote 70 Arusha Region Councils in the Region Council District Councils Code 2001 Arusha City Council 3006 Monduli District Council 3007 Ngorongoro District Council 3084 Karatu District Council 3098 Meru District Council 3099 Arusha District Council 3100 Longido District Council 2 Vote 70 Arusha Region Council Development Budget Summary Local and Foreign 2014/15 Code Council Local Foreign Total 2001 Arusha City Council 8,074,398,000 5,992,812,000 14,067,210,000 3006 Monduli District Council 1,225,860,000 1,325,933,000 2,551,793,000 3007 Ngorongoro District Council 2,242,122,000 1,530,673,000 3,772,795,000 3084 Karatu District Council 1,628,650,000 1,877,664,000 3,506,314,000 3098 Meru District Council 2,736,222,000 1,844,746,000 4,580,968,000 3099 Arusha District Council 3,111,010,000 2,664,172,000 5,775,182,000 3100 Longido District Council 2,088,780,000 1,300,017,000 3,388,797,000 Total 21,107,042,000 16,536,017,000 37,643,059,000 3 Vote 70 Arusha Region Code Description 2012/2013 2013/2014 2014/2015 Actual Expenditure Approved Expenditure Estimates Local Foreign Local Foreign Local Foreign Total Shs. Shs. Shs. 70 Arusha Region 3280 Rural Water Supply & Sanitation 0 2,234,998,000 0 3,914,730,000 0 2,974,627,000 2,974,627,000 4390 Secondary Education Development 0 0 0 1,531,418,000 0 1,401,324,000 1,401,324,000 Programme 4404 District Agriculture Development Support 235,822,000 1,270,729,000 0 0 0 0 0 4457 Rural Financial Services Programmes 52,075,000 0 0 0 0 0 0 4486 Agriculture Sector Dev. -

A Case of Maji Ya Chai Community Women Training I
i JANJA STOVE RENEWABLE SOURCE OF ENERGY FOR SUSTAINABLE ECONOMIC DEVELOPMENT: A CASE OF MAJI YA CHAI COMMUNITY WOMEN TRAINING IN ARUSHA HARRISON E. CHONJO A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT FOR THE REQUIREMENTS FOR THE DEGREEE OF MASTER IN COMMUNITY ECONOMIC DEVELOPMENT OF THE OPEN UNIVERSITY OF TANZANIA 2014 ii CERTIFICATION I, Dr. SIMON WAANE, certifies that I have read and hereby recommend for the acceptance by the Open University of Tanzania (OUT) a project entitled, Janja Stove Renewable Source Of Energy For Sustainable Economic Development: Maji Ya Chai Community Women Training In Arusha in partial fulfillment of the requirements for the degree of Master of Community Economic Development of the Open University of Tanzania. ______________________________________ Dr. Simon Waane (Supervisor) ________________________________ Date iii COPYRIGHT All rights reserved. No part of this work may be printed, reproduced, stored in a retrieval system or transmitted in any form or by any means including photocopying or storing it in any medium by any electronic means and whether or not transiently or incidentally to some other use of this work without the prior permission of the researchers or Open University of Tanzania. iv DECLARATION I, Harrison E. Chonjo hereby declare that this project is my own original work and that it has never been submitted for the similar degree in any other University. ______________________________________ Signature ______________________________________ Date v DEDICATION To my beloved family, My Aunt Agnes A. Chonjo, my young sister Beatrice Mosha, Glory Chonjo and Lightness Chonjo, my niece Michelle, and my parents Mr. & Mrs. Elisonguo Chonjo and my brother Michael, Silas and Sifaeli and Prosper,. -

Meru District Council
THE UNITED REPUBLIC OF TANZANIA PRESIDENT’S OFFICE REGIONAL ADMINISTRATION AND LOCAL GOVERNMENT MERU DISTRICT COUNCIL DRAFT MEDIUM TERM STRATEGIC PLAN 2016/2017 – 2020/2021 DISTRICT EXECUTIVE DIRECTOR, P. O. BOX 462, USA RIVER - ARUSHA TEL: +255 254 1112 FAX: +255 254 1112 E-MAIL: [email protected] WEBSITE: merudc.go.tz USA RIVER FEBRUARY, 2017 Table of Contents PREFACE ............................................................................................................................ iv EXECUTIVE SUMMARY ....................................................................................................... v CHAPTER ONE ............................................................................................................ 1 INTRODUCTION.......................................................................................................... 1 1.1 Contextual Background and Rationale ............................................................. 1 1.2 Methodology .................................................................................................... 1 1.3 Purpose of the Plan .......................................................................................... 1 1.4 Layout of the Corporate Strategic Plan ............................................................ 1 CHAPTER TWO ........................................................................................................... 3 SITUATION ANALYSIS ................................................................................................. 3 2.1 Background -

Tanzania Ecoboma
TANZANIA ECOBOMA A Climate Resilient Model for Maasai Steppe Pastoralists ECOBOMA, a Climate Resilient BACKGROUND 2019 Model for Maasai Steppe Pastoral- The livelihoods of pastoralists in dryland ists is one of five projects, which areas depends entirely on the availability of fragile ecosystem services. In the Maasai ARUSHA falls under the European Union Steppe, there is clear evidence that climate funded Global Climate Change Al- change has already dramatically affected liance. The project encompasses the ecosystem. This is supported by data Zanzibar SEPTEMBER showing a marked decrease in rainfall over the eco-village approach, and has the last 20 years, which has coincided with DAR ES SALAAM made strides to increase and di- a deterioration of the living conditions versify incomes, and strengthen of pastoralists and high vulnerability of livestock herds. Northern Tanzania resilience and reduce vulnerabil- continues to experience unpredictable ity to climate change. The target- weather patterns with serious drought in ed 2000 households are located 2016-2017, followed by severe flooding in 2018 and drought again in 2019. in Arumeru District and depend MAIN ISSUES in water management through newly on the ecosystem for their liveli- established technical committees. Quality hoods, which are increasingly be- • Intense grazing and a decrease in assessments and the ecological monitoring coming threatened due to climate suitable pastures of the rangelands has been conducted with a view to increasing resilience by reducing the change. • Declining water points for humans and livestock vulnerability of the target pastoralist systems. • Increased population within pastoral A group of women and youth have been The project has contributed to societies trained to become leather artisans.