Rounding up Lutetium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

The Rare Earths II
Redis co very of the Elements The Ra re Earth s–The Con fusing Years I A gallery of rare earth scientists and a timeline of their research I I James L. Marshall, Beta Eta 1971 , and Virginia R. Marshall, Beta Eta 2003 , Department of Chemistry, University of North Texas, Denton, TX 76203-5070, [email protected] The rare earths after Mosander. In the pre - vi ou s HEXAGON “Rediscovery” article, 1p we were introduced to the 17 rare earths, found in the f-block and the Group III chemical family of Figure 1. Important scientists dealing with rare earths through the nineteenth century. Johan Gadolin the Periodic Table. Because of a common (1760 –1852) 1g —discovered yttrium (1794). Jöns Jacob Berzelius (1779 –1848) and Martin Heinrich valence electron configuration, the rare earths Klaproth (1743 –1817) 1d —discovered cerium (1803). Carl Gustaf Mosander (1787 –1858) 1p —discovered have similar chemical properties, and their lanthanum (1839), didymium (1840), terbium, and erbium (1843). Jean-Charles deGalissard Marignac chemical separation from one another can be (1817 –1894) 1o —discovered ytterbium (1878) and gadolinium (1880). Per Teodor Cleve (1840 –1905) 1n — difficult. From preparations of the first two rare discovered holmium and thulium (1879). Lars Fredrik Nilson (1840 –1899) 1n —discovered scandium earth element s—yttrium and ceriu m—the (1879). Paul-Émile Lecoq de Boisbaudran (1838 –1912) —discovered samarium (1879) and dysprosium Swedish chemist Carl Gustaf Mosander (Figure (1886). 1b Carl Auer von Welsbach (1858 –1929) 1c —discovered praseodymium and neodymium (1885); 1, 2) was able to separate four additional ele - co-discovered lutetium (1907). -

GLOBAL RARE-EARTH PRODUCTION: HISTORY and OUTLOOK History – the Discovery
Center for Strategic and International Studies Rare Earth Elements: Geology, Geography, and Geopolitics James B. Hedrick Hedrick Consultants, Inc. U.S. Rare Earths, Inc. Burke, Virginia December 15, 2010 Washington, DC GLOBAL RARE-EARTH PRODUCTION: HISTORY AND OUTLOOK History – The Discovery . The rare earths were discovered in 1787 by Swedish Army Lieutenant Carl Axel Arrhenius . Carl, an amateur mineralogist collected the black mineral ytterbite, later renamed gadolinite, from a small feldspar and quartz mine at Ytterby, Sweden . With similar chemical structures the rare earths proved difficult to separate . It was not until 1794 that Finnish chemist Johann Gadolin separated the first impure yttrium oxide from the mineral ytterbite History – The Discovery History – The Commercialization . The rare earths were commercialized when the incandescent lamp mantle industry was established in 1884 with mantles of zirconium, lanthanum, and yttrium oxides with later improvements requiring only the oxides of thorium and cerium. The lamp mantle was discovered by Baron Carl Auer von Welsbach . The mantles also used small amounts of neodymium and praseodymium as an indelible brand name Welsbach label History – The Early Mining . Rare earths were first produced commercially in the 1880s with the mining in Sweden and Norway of the rare-earth thorium phosphate mineral monazite . Foreign Production Brazil produced monazite as early as 1887 India produced monazite starting in 1911 . Domestic Production Monazite production in the United States was first recorded in 1893 in North Carolina - a small tonnage of monazite was reportedly mined in 1887 Monazite mining in South Carolina began in 1903 History – The Processing . Three main methods for separating and refining the rare- earth elements since they were discovered . -

Producers Case Study the Changing
Producers Case Study The Changing Geography of Rare Earth Element Production Introduction The locations where rare earth elements are produced changed repeatedly throughout the 1900s and early 2000s. This variation suggests that production is not determined primarily by the geographic location of rare earth ores. Instead, the location of mines and separation plants is driven by a combination of market prices, government policies, and the actions of producers and manufacturers. As a result where and how rare earth elements are produced could change in the future. Initial Rare Earth Metal Production Although a mineral containing rare earth elements was identified in Sweden in 1788, it took more than 100 years for the first significant industrial product to be made using the rare earths. In the 1890s chemist Carl Auer von Welsbach developed a mantle made of a mixture of 99% thorium and 1% cerium for use with gas streetlights. More than five billion mantles were sold worldwide through the 1930s. Welsbach also developed mischmetal, a mixture of rare earth elements cerium, lanthanum, neodymium, and praseodymium. When alloyed with iron, mischmetal produced a metal that sparked when struck. It was widely used in pocket cigarette lighters as well as automobile ignition switches. The Welsbach Company mined rare earth deposits located in coastal sands in Brazil, India, and North Carolina. India’s coastal sands are also rich in the radioactive element thorium, which is not a rare earth element. India banned the export of these sands in 1948 to preserve its thorium supply for a potential nuclear energy program. At that time the price of rare earth metals spiked until newly established mining locations briefly made South Africa the world’s leading exporter of rare earth ores in the 1950s. -

Rounding up Lutetium Lars Öhrström Suspects That As Time Goes By, We May See More of Lutetium — the Last of the Lanthanoids
in your element Rounding up lutetium Lars Öhrström suspects that as time goes by, we may see more of lutetium — the last of the lanthanoids. e’ll always have Paris” Rick says motexafin (based on the ‘texaphyrins’), a “ to Ilsa in their final goodbye on sub-class of porphyrin-like macrocycles Wthe foggy airstrip of Casablanca with five instead of four nitrogen atoms in in the eponymous film. However, the an approximately planar ring. Motexafin question among chemists about the element lutetium, which features Lu3+ and two lutetium, named after Lutetia, as the French acetate counter-ions coordinated on either capital was known in Roman times, is not so side of the macrocycle, is potentially a good much about having it (it is more abundant photosensitizer in dynamic phototherapy than silver in the Earth’s crust), but rather and has been going through phase I trials where to place it on the map. against prostate cancer2. With its valence electron configuration Uses of the naturally occurring element [Xe]4f 146s25d1, element 71 seems to belong are otherwise scarce, but its isotope Lu-177 is to group 3, but we often see it placed at successfully used in experimental and clinical BRITTA LANGEN, BRITTA SWEDEN UNIVERSITY OF GOTHENBURG, the very end of the lanthanoid series. Its treatments against some severe cancers by downstairs neighbour lawrencium, for which Illustration of a radiolabelled somatostatin hooking it up to a tetraazacyclododecane- experimental data are much more difficult to analogue built using PyMOL (https://pymol.org) tetraacetate (DOTA) ligand grafted to obtain, is in the same ambiguous situation. -

RBRC-32 BNL-6835.4 PARITY ODD BUBBLES in HOT QCD D. KHARZEEV in This ~A~Er We Give a Pedawwicalintroduction~0 Recent Work Of
RBRC-32 BNL-6835.4 PARITY ODD BUBBLES IN HOT QCD D. KHARZEEV RIKEN BNL Research Center, Br$ookhauenNational Laboratory, . Upton, New York 11973-5000, USA R.D. PISARSKI Department of Physics, Brookhaven National Laboratoy, Upton, New York 11973-5000, USA M.H.G. TYTGAT Seruice de Physique Th&orique, (7P 225, Uniuersitc4Libre de Bruzelles, B[ud. du !t%iomphe, 1050 Bruxelles, Belgium We consider the topological susceptibility for an SU(N) gauge theory in the limit of a large number of colors, N + m. At nonzero temperature, the behavior of the topological susceptibility depends upon the order of the reconfining phrrse transition. The meet interesting possibility is if the reconfining transition, at T = Td, is of second order. Then we argue that Witten’s relation implies that the topological suscepti~lfity vanishes in a calculable fdion at Td. Ae noted by Witten, this implies that for sufficiently light quark messes, metaetable etates which act like regions of nonzero O — parity odd bubbles — can arise at temperatures just below Td. Experimentally, parity odd bubbles have dramatic signature% the rI’ meson, and especially the q meson, become light, and are copiously produced. Further, in parity odd bubbles, processes which are normally forbidden, such as q + rr”ro, are allowed. The most direct way to detect parity violation is by measuring a parity odd global seymmetry for charged pions, which we define. 1 Introduction In this .-~a~er we give a Pedawwicalintroduction~0 recent work of ours? We I consider an SU(IV) gau”ge t~e~ry in the limit of a large number of colors, N + co, This is, of course, a familiar limit? We use the large N expansion I to investigate the behavior of the theory at nonzero temperature, especially for the topological susceptibility. -

A Historical Geography of Rare Earth Elements: from Discovery to the Atomic Age
The Extractive Industries and Society 2 (2015) 572–580 Contents lists available at ScienceDirect The Extractive Industries and Society journal homepage: www.elsevier.com/locate/exis Review article A historical geography of rare earth elements: From discovery to the atomic age Julie Michelle Klinger* Frederick S. Pardee School of Global Studies, Boston University, United States ARTICLE INFO ABSTRACT Article history: This article presents a historical geography of rare earth elements from their discovery to the atomic age Received 16 January 2015 with a focus on the period between 1880 and 1960 in order to lend greater depth to the growing body of Received in revised form 19 May 2015 scholarship on the relationship between rare earth elements and global political change. Drawing on Available online 7 July 2015 archival and field research undertaken in the United States, China, Brazil, and Germany between 2011 and 2014, this article advances the following argument. Rare earth elements, and the production of geological Keywords: knowledge about them, have entangled with contentious politics since their first industrial applications Rare earth elements in the late 19th century. The historical geography of rare earth exploration and extraction is defined by a Historical geography fundamental tension between the military-industrial necessity of these elements and the hazards Geology fi Politics associated with their production. This tension played a de nitive role in international colonial, Cold War, Cold war and atomic politics. ã 2015 Elsevier Ltd. All rights reserved. Contents 1. Introduction . ................................................................................................... 572 2. Discovery and classification .......................................................................................... 573 3. Geology, territory, and power . ..................................................................................... 573 4. The political life of rare earth elements ............................................................................... -

Consumers Case Study
Consumers Case Study Can Consumer Choices Make Rare Earth Production More Sustainable? Introduction Today the rare earth elements are used in many products that benefit consumers (people or organizations that buy manufactured products). The rare earths are often an “invisible” ingredient in such products, however, and manufacturers rarely advertise which metals they use in production. As a consequence many consumers are not aware of how their purchases are connected to the rare earth industry. Some activists hope to change this. They want consumers to better understand how buying certain products is connected to negative environmental impacts, low wages, and unsafe conditions for people living and working in distant countries. They want consumers to see their purchasing choices as political acts. By helping people become “ethical consumers,” these activists hope to use the power of markets to protect the environment and ensure that workers get a fair share of the benefits of production. One strategy for encouraging ethical consumption is certification, a kind of “seal of approval” for products made in ways that are more environmentally sustainable or that treat workers more fairly. History of Consumer Products with Rare Earth Elements Cigarette spark lighters were the first significant consumer product to use rare earth elements. In the early 1900s chemist Carl Auer von Welsbach developed metal alloys he named “ferrocerium” and “mischmetal” that combined iron with various rare earth elements. These alloys sparked with intense heat when struck, and the sparks then ignited a liquid fuel such as kerosene. More products made with rare earths arrived in American living rooms and garages in the decades after World War II. -

THE MAJOR RARE-EARTH-ELEMENT DEPOSITS of AUSTRALIA: GEOLOGICAL SETTING, EXPLORATION, and RESOURCES Figure 1.1
CHAPTER ONE WHAT ARE RARE- EARTH ELEMENTS? 1.1. INTRODUCTION latter two elements are classified as REE because of their similar physical and chemical properties to the The rare-earth elements (REE) are a group of seventeen lanthanides, and they are commonly associated with speciality metals that form the largest chemically these elements in many ore deposits. Chemically, coherent group in the Periodic Table of the Elements1 yttrium resembles the lanthanide metals more closely (Haxel et al., 2005). The lanthanide series of inner- than its neighbor in the periodic table, scandium, and transition metals with atomic numbers ranging from if its physical properties were plotted against atomic 57 to 71 is located on the second bottom row of the number then it would have an apparent number periodic table (Fig. 1.1). The lanthanide series of of 64.5 to 67.5, placing it between the lanthanides elements are often displayed in an expanded field at gadolinium and erbium. Some investigators who want the base of the table directly above the actinide series to emphasise the lanthanide connection of the REE of elements. In order of increasing atomic number the group, use the prefix ‘lanthanide’ (e.g., lanthanide REE: REE are: lanthanum (La), cerium (Ce), praseodymium see Chapter 2). In some classifications, the second element of the actinide series, thorium (Th: Mernagh (Pr), neodymium (Nd), promethium (Pm), samarium 1 (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), and Miezitis, 2008), is also included in the REE dysprosium (Dy), holmium (Ho), erbium (Er), thulium group, while promethium (Pm), which is a radioactive (Tm), ytterbium (Yb), and lutetium (Lu). -

The Course of Hermann Aron's
MAX-PLANCK-INST I T U T F Ü R W I SSENSCHAFTSGESCH I CHTE Max Planck Institute for the History of Science 2009 PREPRINT 370 Shaul Katzir From academic physics to invention and industry: the course of Hermann Aron’s (1845–1913) career Shaul Katzir From academic physics to invention and industry: the course of Hermann Aron’s (1845-1913) career Hermann Aron had an unusual career for a German physicist of the Imperial era. He was an academic lecturer of physics, an inventor, a founder and the manager of a company for electric devices, which employed more than 1,000 employees. Born in 1845 to a modest provincial Jewish family, Aron went through leading schools of the German educational system, received a doctorate in physics and in 1876 the venia legendi - teaching privilege, with which he became a Privatdozent (lecturer) at Berlin university. However, rather than becoming a university professor - the desired goal of this career track - he turned to technology and then industry with the invention of an electricity meter and the foundation of a successful company for its development and manufacture in 1883. By 1897 he owned four sister companies in Berlin, Paris, London, and Vienna-Budapest and manufacturing factories in these cities as well as in Silesia. While in the twentieth century such a career trajectory of a university teacher was not the common case, it was even less so in the late nineteenth century in physics; in many respects it was unique. Aron’s was not the case of a newly qualified doctor hired by industry. -

United States Patent Office
UNITED STATES PATENT OFFICE. CARL AUER VON WELSBACH, OF VIENNA, AUSTRIA-HUNGARY, ASSIGNOR TO THE WELSBACH INCANDESCENT GAS LIGHT COMPANY, OF NEWJERSEY. GAS IN CAND ESCENT. SPECIFICATION forming part of Letters Patent No. 403,803, dated May 21, 1889. Application filed March 31, 1888, Serial No. 269,205. (No specimens.) Patented in France November 4, 1885, No. 172,064 in Bel gium November 4, 1885, No. 70,739; in England March 13, 1886, No, 3,592 in Sweden April 14, 1886, No. 687 in Germany April 29, 1886, No. 41,945; in Finland July 10, 1886, No. 261 in Norway August 25, 1886, No. 88; in Italy October 13, 1886, XL 415 in Portugal April 6, 1887, No. 1,127 in Russia, December 31, 1887, No, l2,505, and in Austria-Hungary March 28, 1888, No. 44,989 and No. 5,176, To all whom it may concern: thorium oxide and erbium oxide, though the Be it known that I, CARLAUER VON WELS thorium oxide may be partly replaced by Zir BACH, a subject of the Emperor of Austria, conium oxide. All the above-named bodies residing at Vienna, Austria, have invented may be mixed together in varying propor- 5o 5 new and useful Improvements in Gas-Incan tions to produce green light of differing descents, (for which I have obtained Letters shades. A solution is made of the Selected Patent in Great Britain, dated March 13, 1886, elements in the form of a salt-preferably the No. 3,592; Sweden, dated April 14, 1886, No. nitrate or acetate-and with this solution the 687; Norway, dated August 25, 1886, No. -
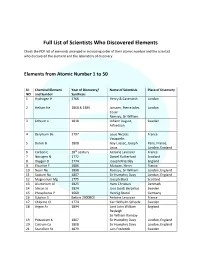
Full List of Scientists Who Discovered Elements
Full List of Scientists Who Discovered Elements Check the PDF list of elements arranged in increasing order of their atomic number and the scientist who discovered the element and the laboratory of discovery. Elements from Atomic Number 1 to 50 SI. Chemical Element Year of Discovery/ Name of Scientists Place of Discovery NO and Symbol Synthesis 1 Hydrogen H 1766 Henry & Cavendish London 2 Helium He 1868 & 1895 Janssen, Pierre Jules London Cesar Ramsay, Sir William 3 Lithium Li 1818 Johann August, Sweden Arfvedson 4 Beryllium Be 1797 Louis Nicolas France Vauquelin 5 Boron B 1808 Gay-Lussac, Joseph Paris, France, Louis London, England 6 Carbon C 18th century Antoine Lavoisier France 7 Nitrogen N 1772 Daniel Rutherford Scotland 8 Oxygen O 1774 Joseph Priestley England 9 Fluorine F 1886 Moissan, Henri France 10 Neon Ne 1898 Ramsay, Sir William London, England 11 Sodium Na 1807 Sir Humphry Davy London, England 12 Magnesium Mg 1775 Joseph Black Scotland 13 Aluminium Al 1825 Hans Christian Denmark 14 Silicon Si 1824 Jons Jacob Berzelius Sweden 15 Phosphorus P 1669 Hennig Brand Germany 16 Sulphur S Before 2000BCE Antoine Lavoisier France 17 Chlorine Cl 1774 Carl Wilhelm Scheele Sweden 18 Argon Ar 1894 Lord John William England Rayleigh Sir William Ramsay 19 Potassium K 1807 Sir Humphry Davy London, England 20 Calcium Ca 1808 Sir Humphry Davy London, England 21 Scandium Sc 1879 Lars Frederick Sweden 22 Titanium Ti 1791, 1795 William Gregor England, Germany Martin Heinrich 23 Vanadium V 1801 Andrés Manuel Mexico 24 Chromium Cr 1797 Louis-Nicholas