Nucleic Acids and Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -

Oligomeric A2 + B3 Approach to Branched Poly(Arylene Ether Sulfone)
“One-Pot” Oligomeric A 2 + B 3 Approach to Branched Poly(arylene ether sulfone)s: Reactivity Ratio Controlled Polycondensation A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By ANDREA M. ELSEN B.S., Wright State University, 2007 2009 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES June 19, 200 9 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Andrea M. Elsen ENTITLED “One-Pot” Oligomeric A2 + B3 Approach to Branched Poly(arylene ether sulfone)s: Reactivity Ratio Controlled Polycondenstation BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science . _________________________ Eric Fossum, Ph.D. Thesis Director _________________________ Kenneth Turnbull, Ph.D. Department Chair Committee on Final Examination ____________________________ Eric Fossum, Ph.D. ____________________________ Kenneth Turnbull, Ph.D. ____________________________ William A. Feld, Ph.D. ____________________________ Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies Abstract Elsen, Andrea M. M.S., Department of Chemistry, Wright State University, 2009. “One-Pot” Oligomeric A 2 + B 3 Approach to Branched Poly(arylene ether sulfone)s: Reactivity Ratio Controlled Polycondensation The synthesis of fully soluble branched poly(arylene ether)s via an oligomeric A 2 + B 3 system, in which the A 2 oligomers are generated in situ, is presented. This approach takes advantage of the significantly higher reactivity toward nucleophilic aromatic substitution reactions, NAS, of B 2, 4-Fluorophenyl sulfone, relative to B 3, tris (4-Fluorophenyl) phosphine oxide. The A 2 oligomers were synthesized by reaction of Bisphenol-A and B 2, in the presence of the B 3 unit, at temperatures between 100 and 160 °C, followed by an increase in the reaction temperature to 180 °C at which point the branching unit was incorporated. -
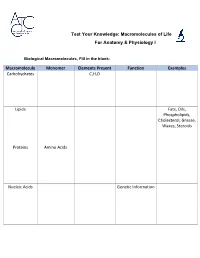
Macromolecules of Life Worksheet
Test Your Knowledge: Macromolecules of Life For Anatomy & Physiology I Biological Macromolecules, Fill in the blank: Macromolecule Monomer Elements Present Function Examples Carbohydrates C,H,O Lipids Fats, Oils, Phospholipids, Cholesterol, Grease, Waxes, Steroids Proteins Amino Acids Nucleic Acids Genetic Information Carbohydrates are classified by __________. The most common simple sugars are glucose, galactose and fructose that are made of a single sugar molecule. These can be classified as __________. Sucrose and __________ are classified as disaccharides; they are made of two monosaccharides joined by a dehydration reaction. The most complex carbohydrates are starch, __________ and cellulose, classified as __________. Lipids most abundant form are __________. Triglycerides building blocks are 1 __________ and 3 __________ per molecule. If a triglyceride only contains __________ bonds that contain the maximum number of __________, then it is classified as a saturated fat. If a triglyceride contains one or more __________ bonds, then it is classified as an unsaturated fat. Lipids are also responsible for a major component of the cell membrane wall that is both attracted to and repelled by water, called __________. The tail of this structure is made of 2 __________, that are water insoluble (hydrophobic). The head of this structure is made of a single __________, that is water soluble (hydrophilic). Proteins building blocks are amino acids that are held together with __________ bonds. These are covalent bonds that link the amino end of one amino acid with the carboxyl end of another. Their overall shape determines their __________. The complex 3D shape of a protein is called a __________. -

Specifications Guide Americas Petrochemicals Latest Update: July 2020
Specifications Guide Americas Petrochemicals Latest update: July 2020 Definitions of the trading locations for which Platts publishes daily indexes or assessments 2 Olefins 3 US aromatics 6 Latin American aromatics 8 US polymers 10 Latin American polymers 13 US intermediates 16 US hydrocarbon solvents 17 US chlor alkali 18 US oxygenated solvents 19 Liquid and gas chemical freight 21 Global petrochemical indices 22 Revision history 23 www.spglobal.com/platts Specifications Guide Americas Petrochemicals: July 2020 DEFINITIONS OF THE TRADING LOCATIONS FOR WHICH PLATTS PUBLISHES DAILY INDEXES OR ASSESSMENTS The following specifications guide contains the primary specifications for S&P Global Platts petrochemical assessments in the Americas. All the assessments listed here employ Platts Assessments Methodology, as published at https://www.spglobal.com/platts/plattscontent/_assets/_files/en/our-methodology/methodology-specifications/platts-assessments-methodology-guide.pdf. These guides are designed to give Platts subscribers as much information as possible about a wide range of methodology and specification questions. This guide is current at the time of publication. Platts may issue further updates and enhancements to this methodology and will announce these to subscribers through its usual publications of record. Such updates will be included in the next version of this guide. Platts editorial staff and managers are available to provide guidance when assessment issues require clarification. OLEFINS Assessment CURRENCY CODE Mavg Wavg TYPE -

Guidance for Monomers and Polymers
GUIDANCE Guidance for monomers and polymers April 2012 Version 2.0 Guidance for the implementation of REACH Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu Guidance for monomers and polymers 2 Version 2.0 April 2012 Version Changes Date Version 0 First edition June 2007 Version 1 Section 2.2 - More explanations given on the 18/03/2008 definition of polymer (including different types of additives). Most of section 3.3 transferred to here. Section 3.1 - Clarification of cases where the substance is used both as monomer and as intermediate under strictly controlled conditions. Section 3.2.1.1 - Addition of a sentence to clarify that there is no need to register stabilisers Section 3.2.1.2 - The section has been modified in order to reflect a proposal for solution for those substances already notified. Section 3.2.1.3 - Some wording change for clarification that only the substance used for the modification of the natural polymer needs to be registered when ending up chemically bound to the polymer. Section 3.2.1.4 - Need for update acknowledged. Previous Section 3.3 - Deleted and mostly transferred to section 2.2. Version 1.1 Section 3.2.1.2 - Based on the comments 27/05/2008 received from Ireland after the CA meeting in December 2007 some additional guidance on what needs to be done for notified polymers has been added (4 pages). Version 2.0 Section 2.1 and 3.1 – Reference to monomers as April 2012 intermediate reworded in order to be consistent with new clarification of intermediate definition. -

Molecular Biology and Applied Genetics
MOLECULAR BIOLOGY AND APPLIED GENETICS FOR Medical Laboratory Technology Students Upgraded Lecture Note Series Mohammed Awole Adem Jimma University MOLECULAR BIOLOGY AND APPLIED GENETICS For Medical Laboratory Technician Students Lecture Note Series Mohammed Awole Adem Upgraded - 2006 In collaboration with The Carter Center (EPHTI) and The Federal Democratic Republic of Ethiopia Ministry of Education and Ministry of Health Jimma University PREFACE The problem faced today in the learning and teaching of Applied Genetics and Molecular Biology for laboratory technologists in universities, colleges andhealth institutions primarily from the unavailability of textbooks that focus on the needs of Ethiopian students. This lecture note has been prepared with the primary aim of alleviating the problems encountered in the teaching of Medical Applied Genetics and Molecular Biology course and in minimizing discrepancies prevailing among the different teaching and training health institutions. It can also be used in teaching any introductory course on medical Applied Genetics and Molecular Biology and as a reference material. This lecture note is specifically designed for medical laboratory technologists, and includes only those areas of molecular cell biology and Applied Genetics relevant to degree-level understanding of modern laboratory technology. Since genetics is prerequisite course to molecular biology, the lecture note starts with Genetics i followed by Molecular Biology. It provides students with molecular background to enable them to understand and critically analyze recent advances in laboratory sciences. Finally, it contains a glossary, which summarizes important terminologies used in the text. Each chapter begins by specific learning objectives and at the end of each chapter review questions are also included. -

The Structure and Function of Large Biological Molecules 5
The Structure and Function of Large Biological Molecules 5 Figure 5.1 Why is the structure of a protein important for its function? KEY CONCEPTS The Molecules of Life Given the rich complexity of life on Earth, it might surprise you that the most 5.1 Macromolecules are polymers, built from monomers important large molecules found in all living things—from bacteria to elephants— can be sorted into just four main classes: carbohydrates, lipids, proteins, and nucleic 5.2 Carbohydrates serve as fuel acids. On the molecular scale, members of three of these classes—carbohydrates, and building material proteins, and nucleic acids—are huge and are therefore called macromolecules. 5.3 Lipids are a diverse group of For example, a protein may consist of thousands of atoms that form a molecular hydrophobic molecules colossus with a mass well over 100,000 daltons. Considering the size and complexity 5.4 Proteins include a diversity of of macromolecules, it is noteworthy that biochemists have determined the detailed structures, resulting in a wide structure of so many of them. The image in Figure 5.1 is a molecular model of a range of functions protein called alcohol dehydrogenase, which breaks down alcohol in the body. 5.5 Nucleic acids store, transmit, The architecture of a large biological molecule plays an essential role in its and help express hereditary function. Like water and simple organic molecules, large biological molecules information exhibit unique emergent properties arising from the orderly arrangement of their 5.6 Genomics and proteomics have atoms. In this chapter, we’ll first consider how macromolecules are built. -

Bio-Based and Biodegradable Plastics – Facts and Figures Focus on Food Packaging in the Netherlands
Bio-based and biodegradable plastics – Facts and Figures Focus on food packaging in the Netherlands Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Rapport nr. 1722 Bio-based and biodegradable plastics - Facts and Figures Focus on food packaging in the Netherlands Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Report 1722 Colophon Title Bio-based and biodegradable plastics - Facts and Figures Author(s) Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Number Wageningen Food & Biobased Research number 1722 ISBN-number 978-94-6343-121-7 DOI http://dx.doi.org/10.18174/408350 Date of publication April 2017 Version Concept Confidentiality No/yes+date of expiration OPD code OPD code Approved by Christiaan Bolck Review Intern Name reviewer Christaan Bolck Sponsor RVO.nl + Dutch Ministry of Economic Affairs Client RVO.nl + Dutch Ministry of Economic Affairs Wageningen Food & Biobased Research P.O. Box 17 NL-6700 AA Wageningen Tel: +31 (0)317 480 084 E-mail: [email protected] Internet: www.wur.nl/foodandbiobased-research © Wageningen Food & Biobased Research, institute within the legal entity Stichting Wageningen Research All rights reserved. No part of this publication may be reproduced, stored in a retrieval system of any nature, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher. The publisher does not accept any liability for inaccuracies in this report. 2 © Wageningen Food & Biobased Research, institute within the legal entity Stichting Wageningen Research Preface For over 25 years Wageningen Food & Biobased Research (WFBR) is involved in research and development of bio-based materials and products. -

Monomers and Polymers Derived from Biological Sources
danimerscientific.com Monomers and Polymers derived from Biological Sources Opportunities and Challenges Overview danimerscientific.com • We are all familiar with the news about plastic waste • Biodegradable is more important than biorenewable • Bio-based plastics cannot sacrifice on performance • Biomass purification versus bio-organism production • Biomass products take existing natural materials and react/purify to obtain specified monomers/polymers • Bio-synthetic routes synthesize monomers or polymers via a metabolic pathway (purification still necessary) • Biodegradable plastics • Microbes in the environment will break down the plastic and use it as a food source • Can occur in many environments including terrestrial and marine • Compostable • Products will degrade in municipal and industrial composting facilities • Require inputs such as moisture and heat to disintegrate materials PLA/PHA danimerscientific.com CH3 (C) H CH H 2 n O H 3 O * ( * O)( O) x 1 PLA PHA - Biodegradable in an industrial compost environment - 100% biodegradable - Will de-polymerize via hydrolysis given enough impetus - Produced via bio-synthesis - Monomer (lactide) produced via bio-synthesis - Micro-organisms create polymers; not monomers - Plastic then produced via polymerization - Polymers then processed/compounded to produce plastics and polymers for specific applications PLA/PHA: Fermentation basics danimerscientific.com - Common PLA pathway is Lactobacillus genus such as L. delbrueckii, L. amylophilus and L. leichmanii1 - Most common monomer produced is L-lactic acid - Corn is converted to corn sugar (starch); commonly dextrose - Starch is then heated with acid or enzymes to completely hydrolyze to dextrose - The dextrose is then subjected to glycolysis to pyruvate, ATP and NADH - The pyruvate is then metabolized to lactate and the lactic acid - PHA is synthesized via microorganism E. -

Emulsion Polymerization
Emulsion Polymerization Reference: Aspen Polymers: Unit Operations and Reaction Models, Aspen Technology, Inc., 2013. 1. Introduction Emulsion polymerization is an industrially important process for the production of polymers used as synthetic rubber, adhesives, paints, inks, coatings, etc. The polymerization is usually carried out using water as the dispersion medium. This makes emulsion polymerization less detrimental to the environment than other processes in which volatile organic liquids are used as a medium. In addition, emulsion polymerization offers distinct processing advantages for the production of polymers. Unlike in bulk or solution polymerization, the viscosity of the reaction mixture does not increase as dramatically as polymerization progresses. For this reason, the emulsion polymerization process offers excellent heat transfer and good temperature throughout the course of polymer synthesis. In emulsion polymerization, free-radical propagation reactions take place in particles isolated from each other by the intervening dispersion medium. This reduces termination rates, giving high polymerization rates, and simultaneously makes it possible to produce high molecular weight polymers. One can increase the rate of polymerization without reducing the molecular weight of the polymer. Emulsion polymerization has more recently become important for the production of a wide variety of specialty polymers. This process is always chosen when the polymer product is used in latex form. 2. Reaction Kinetic Scheme To appreciate the complexities of emulsion polymerization, a basic understanding of the fundamentals of particle formation and of the kinetics of the subsequent particle growth stage is required. A number of mechanisms have been proposed for particle formation. It is generally accepted that any one of the mechanisms could be responsible for particle formation depending on the nature of the monomer and the amount of emulsifier used in the recipe. -

Chapter 2 Introduction to Some Basic Features of Genetic Information
Chapter 2 Introduction to some basic features of genetic information: From DNA to proteins 1 1, 2 1, 3 DAVID QUIST, KAARE M. NIELSEN AND TERJE TRAAVIK 1 THE NORWEGIAN INSTITUTE OF GENE ECOLOGY (GENØK), TROMSØ, NORWAY 2 DEPARTMENT OF PHARMACY, UNIVERSITY OF TROMSØ, NORWAY 3 DEPARTMENT OF MICROBIOLOGY AND VIROLOGY, UNIVERSITY OF TROMSØ, NORWAY Molecular biology is the study of biology at a molecular level, with the aim of understanding the interactions between the various systems of a cell, including the interrelationship and regulation of DNA, RNA and protein synthesis. In general terms, DNA (deoxyribonucleic acid) is the basic genetic information macromolecule of the cell. It provides the rudimentary instructions for all kinds of biochemical functions, from making proteins to regulatory functions. DNA is found in every cell and every cell type and organism, from single-celled organisms (prokaryotes, e.g. bacteria), to larger multicellular organisms (eukaryotes, e.g. seaweeds, fungi, plant, animals) that can have many different cell and tissue types.1 DNA contains the genetic ‘code’ of information that makes each species unique. Smaller variations in the DNA can lead to minor differences among individuals of the same species. The combination of specific DNA composition, epigenetic changes (see Chapter 8) and environmental influences determine an organism’s appearance and development. In this book, we discuss how the main carrier of heritable information (DNA) and the environment interact, with particular emphasis on how genetic engineering may intentionally or unintentionally affect this interaction. This chapter focuses on DNA, RNA and the concept of genes. It is structured as follows: 1. -

Monomers – Styrene and Vinyl Chloride
Chemical Information Sheet Version 2.0 | March 2021 MONOMERS – STYRENE AND VINYL CHLORIDE Other Names Styrene: Ethenylbenzene, vinylbenzene, Monomers are chemical precursors that link together to phenylethene create polymer materials. Styrene and vinyl chloride are Vinyl Chloride: VCM, chloroethene monomers that may be present in low concentrations in some polymer materials. The presence of these monomers can be related to the process controls during CAS Number Substance polymer production. 100-42-5 Styrene Uses in the Supply Chain 75-01-4 Vinyl Chloride Styrene is a colorless liquid that evaporates easily which may be used to create polymers including polystyrene, ABS plastic, May Be Found In Styrene: Polystyrene, Acrylonitrile-butadiene- synthetic rubber (SBR) and other materials. Styrene can also be styrene (ABS) plastic, Styrene-butadiene rubber (SBR), styrene-divinylbenzene (S-DVB) used in plastic packaging and electrical parts. Vinyl Chloride: Polyvinyl chloride (PVC), Vinyl Chloride is used in production of polyvinyl chloride vinyl polymers, plastisol screen prints, plastic (PVC) and vinyl polymers, which can be hard or flexible parts, coatings for leather, synthetic leather and materials. PVC can be associated with plastisol screen prints, textiles plastic parts, and a variety of coatings on leather, synthetic leather, and textiles. Why Monomers Are Restricted ▪ Legislation in major markets globally restricts or regulates the presence of styrene and vinyl chloride in finished products or materials. ▪ Monomers can present a variety