Redalyc.Smallpox Eradication and Brazil: an Interview with Donald A. Henderson
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Independent Monitoring Board of the Global Polio Eradication Initiative Report
Independent Monitoring Board of the Global Polio Eradication Initiative Report July 2011 Independent This second report follows our third meeting, held in London on 30 June and 1 July 2011. Monitoring Board of the At present the needs are: Global Polio • To concisely understand the global situation Eradication Initiative • To maintain clear and energetic focus in each country with ongoing transmission July 2011 • To see the wood for the trees in identifying and dealing with the programme’s key risks • To find innovative solutions that break through entrenched problems • To surface fundamental problems that need urgent attention We are grateful to the many partners of the Global Polio Eradication Initiative who have provided us with information, logistical support, and valuable insights. We are grateful for their help, and commend their commitment. Our role remains to speak with a clear, objective voice that is independent of any of these partners. We each sit on this board in a personal capacity. We remain resolutely independent, and will continue to present our frank view without fear or favour. Sir Liam Donaldson (Chair) Dr Mushtaque Chowdhury* Former Chief Medical Officer, England Associate Director, Rockefeller Foundation Dr Nasr El Sayed Dr Ciro de Quadros Assistant Minister of Health, Egypt Executive Vice President, Sabin Vaccine Institute Dr Jeffrey Koplan Dr Sigrun Mogedal Vice President for Global Health Special Advisor, Norweigan Knowledge Centre Director, Emory Global Health Institute for the Health Services Professor Ruth Nduati Dr Arvind Singhal Chairperson, Department of Paediatrics and Child Health Marston Endowed Professor of Communication University of Nairobi University of Texas at El Paso Professor Michael Toole Head, Centre for International Health *Dr Chowdhury was unable to participate in the meeting but Burnet Institute, Melbourne endorses this report The Independent Monitoring Board was convened at the request of the World Health Assembly to monitor and guide the progress of the Global Polio Eradication Initiative’s 2010-12 Strategic Plan. -

THE ERADICATION of POLIOMYELITIS (Fhe Albert V.• Sabin Lecture)
THE ERADICATIONOF POLIOMYELITIS (fhe Albert V.•Sabin Lecture) by Donald Henderson, M.D., M.P.H. University Distinguished Service Professor The JohnsHopkins University Baltimore, Maryland 21205 Cirode Quadros, M.D., M.P.H. Regional Advisor Expanded Programme on lmmunii.ation Pan American Health Organization 525 23rd Street, N. W. Washington, D.C. 20037 Introduction The understanding and ultimate conquest of poliomyelitis was Albert Sabin's life long preoccupation, beginning with his earliest work in 1931. (Sabin and Olitsky, 1936; Sabin, 1965) The magnitude of that effort was aptly summarized by Paul in his landmark history of polio: "No man has ever contributed so much effective information - and so continuously over so many years - to so many aspects of poliomyelitis." (Paul, 1971) Thus, appropriately, this inaugural Sabin lecture deals with poliomyelitis and its eradication. Polio Vaccine Development and Its Introduction In the quest for polio control and ultimately eradication, several landmarks deserve special mention. At the outset, progress was contingent on the development of a vaccine and the production of a vaccine, in turn, necessitated the discovery of new methods to grow large quantities of virus. The breakthrough occurred in 1969 when Enders and his colleagues showed that large quantities of poliovirus could be grown in a variety of human cell tissue cultures and that the virus could be quantitatively assayed by its cytopathic effect. (Enders, Weller and Robbins, 1969) Preparation of an inactivated vaccine was, in principle, a comparatively straightforward process. In brief, large quantities of virus were grown. then purified, inactivated with formalin and bottled. Assurance that the virus had been inactivated could be demonstrated by growth in tissue. -

Modernization This Ispublic Health: Acanadianhistory
Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History CHAPTER 3 printable version of this chapter Modernization and Growth . 3 .1 Maternal and Child Health . 3 .2 Public Health Nurses . 3 .4 Full-Time Health Units . 3 .5 Services for Indigenous Communities . 3 .7 Venereal Disease . 3 .8 Charitable Organizations . 3 .9 Lapses in Oversight: Smallpox and Typhoid . 3 .11 Toronto’s School of Hygiene and Connaught Laboratories . 3 .14 Poliomyelitis . 3 .15 Depression and the End of Expansion . 3 .16 Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History 3.1 CHAPTER 3 Dominion Council of Health Modernization The .Dominion .Council .of .Health . was .chaired .by .the .federal .Deputy . Minister .of .Health .and .made .up .of . and Growth the .chief .provincial .officers .of .health . and .representatives .of .urban .and . A .new .public .health .order .emerged .in .the .aftermath .of .World .War .I, .represented .at .the . rural .women, .labour, .agriculture .and . universities, .the .latter .representing . international .level .by .the .development .of .the .Health .Organization .of .the .League .of .Nations . academic .and .scientific .expertise .in . In .Canada, .this .new .order .was .symbolized .by .the .Dominion .Council .of .Health .(DCH), .which . medicine, .public .health .and .laboratory . was .created .to .develop .policies .and .advise .the .new .federal .Department .of .Health . .Initially, . research . .The .Council .provided .a . the .Department .was .primarily .focused .on .collecting .and .distributing .information, .with .some . twice-yearly .forum .to .openly .discuss, . lesser .effort .to .develop .federal .laboratory .research .capacity . compare .and .co-ordinate .strategies .on . the .major .public .health .concerns .of .the . -

The Spanish Flu and Canadian Influenza Vaccine Initiatives
DefiningMomentsCanada.ca THE SPANISH FLU AND CANADIAN INFLUENZA VACCINE INITIATIVES Christopher J. Rutty, Ph.D. significant but sometimes overlooked element in the history of the Spanish Flu pandemic of 1918-19 A was the experimental production, distribution and wide use of influenza vaccines. Since the vaccines were based on an erroneous view that influenza was caused by a bacteria (the influenza virus would not be isolated until 1933) such vaccines were ineffective and thus of little importance to the course of the pandemic from a medical or public health perspective. However, the story of the vaccines produced to prevent pandemic influenza, particularly in Canada, reveals much about the application of uncertain knowledge in the face of an unprecedented public health emergency. It also reveals the changing state of Canadian biotechnology capacity at the end of World War I. Connaught Antitoxin Laboratories of the University of Toronto led the most significant initiative to produce an influenza vaccine.1 Connaught’s flu vaccine initiative coincided with a significant production effort by the Ontario Provincial Laboratories, along with various smaller scale and more local efforts, particularly in Kingston and Winnipeg.2 Canadian vaccine efforts were also linked to emergency vaccine preparation in New York City, Boston, and at the Mayo Clinic in Minnesota.3 Influenza vaccines were similarly prepared in other countries in the face of the global pandemic. In 1919, Dr. John G. FitzGerald, Director of Connaught Antitoxin Laboratories, began his summary of Connaught’s influenza vaccine work by observing: “almost coincident with the end of the war a great emergency arose in which the laboratories were provided with an opportunity of doing public service work of a national character.”4 Connaught had been established in May, 1914, as the Antitoxin Laboratory in the Department of Hygiene, 1 Online resources about the history of Connaught Laboratories include: http://connaught.research.utoronto.ca/history/; http:// thelegacyproject.ca 2 J.W.S. -

Correspondence, Research Notes and Papers, Articles
MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology Correspondence, research notes and papers, articles, speeches, travel journals, drawings, and sketches, photographs, clippings, and other memorabilia, awards and prizes. Includes some papers from his widow Henrietta Banting (d. 1976). 1908-1976. Extent: 63 boxes (approx. 8 metres) Part of the collection was deposited in the Library in 1957 by the “Committee concerned with the Banting Memorabilia”, which had been set up after the death of Banting in 1941. These materials included papers from Banting’s office. At the same time the books found in his office (largely scientific and medical texts and journals) were also deposited in the University Library. These now form a separate collection in the Thomas Fisher Rare Book Library. The remainder of the collection was bequeathed to the Thomas Fisher Rare Book Library by Banting’s widow, Dr. Henrietta Banting, in 1976. This part of the collection included materials collected by Henrietta Banting for her projected biography of F.G. Banting, as well as correspondence and memorabilia relating to her won career. Researchers who wish to publish extensively from previously unpublished material from this collection should discuss the question of literary rights with: Mrs. Nancy Banting 12420 Blackstock Street Maple Ridge, British Columbia V2X 5N6 (1989) Indicates a letter of application addressed to the Director, Thomas Fisher Rare Book Library, is needed due to fragility of originals or confidential nature of documents. 1 MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology 1891 FGB born in Alliston, Ont. To Margaret (Grant) and William Thompson Banting. -
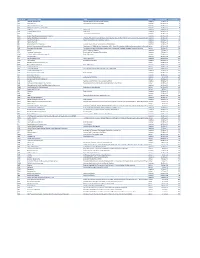
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

Leadership in Global Health: the Case of Ciro De Quadros, a Testament to Values, Valor, and Vision
Pan American Journal Profile of Public Health When Dr. Bill Foege wrote “When Words Fail,” he was referring to how difficult Leadership in it was for him to describe adequately, in written words, all the effort that is involved from the scientific conceptualization of a new vaccine, to eventual bench discovery global health: and development, to the training and supply chain logistics, and ultimately to the moment the needle pricks the skin to save a life of a child (1). He called it the “chain the case of Ciro of perfection”. He was “at a loss” to describe this cascade of events with due justice. I have been asked to describe the leadership attributes of Dr. Ciro de Quadros as a de Quadros, a case study in best practice and lessons to be learned. Similarly, I too am at a loss. Simply put, Ciro broke the mold. testament to I should disclose that what I am about to write is influenced by decades of either working directly for Ciro, as was the case during the polio eradication era in the Americas, to collaborating with Ciro more recently on various projects that in- values, valor, cluded measles and rubella elimination, the introduction of new vaccines, and sur- veillance of infectious diseases. The work evolved into a relationship that bridged and vision from professional mentorship, to actual friends with a deep sense of admiration, love, and mutual respect. In global health, leadership is somewhat like the “self-actualization” of public Jon Kim Andrus1 health practice. The “leader” must have all those attributes that embodies a fully realized individual, positioned in the system to make things happen, such as in Ciro’s case, to help create a world of equitable access to life-saving vaccines. -

Chronological Introduction of Types of Vaccine Products That Are Still Licensed in the United States
Appendix 2.3 CHRONOLOGICAL INTRODUCTION OF TYPES OF VACCINE PRODUCTS THAT ARE STILL LICENSED IN THE UNITED STATES The year of introduction of each of 49 of the 51 shown in table 2.3B, American pharmaceutical com- types of vaccine prducts currently licensed in the panies were issued 37 (89 percent) of the original United States, alolng with the manufacturing estab- licenses for these 42 products. New or improved lishment with the oldest license still in effect for each types of products that are currently licensed have product, is shown in table 2.3 A.] For 42 (86 percent) been introduced at a fairly consistent rate of three to of these 49 products, the establishment that received seven products per each 5-year interval since 1940.2 the original product license still holds this license. As Ten of the currently licensed products were licensed before 1940. Table 2.3A—Chronological Introduction of Types of Vaccine Products Still Licensed in the United States Year Type of vaccine product Establishment with oldest product license still in effecta 1903 Dlphtherla antitoxin Massachusetts Public Health Biologic Laboratories (191 7) 1907 Tetanus antitoxin Parke. Davis and Company (191 5) 1914 Pertussis vaccine Lederle Laboratories Typhoid vaccine Massachusetts Public Health Biologic Laboratories (191 7) Rabies vaccine . Eli Lilly and Company* 1917 Cholera vaccine Eli Lilly and Company* 1926 Diphtheria toxoid Parke, Davis and Company (1927) 1933 Staphylococcus toxoid Lederle Laboratories* Tetanus toxoid Merck Sharp and Dohme 1941 Typhus vaccine Eli Lilly and Company 1942 Plague vaccine .., . Cutter Laboratories* Rocky Mountain Spotted Fever vaccine. Lederle Laboratories* 1945 InfIuenza virus vaccine Lederle Laboratories Merck Sharp and Dohme Parke, Davis and Company * 1946 Parke, Davis and Company ● 1947 Parke, Davis and Company (1949) 1948 Parke, Davis and Company (1952) Parke. -

2010 Albert B. Sabin Gold Medal Awarded to Dr. John D. Clemens
Home About Us Press Room Support Sabin Michael Marine May 2010 Appointed CEO of The Sabin Report | Volume 12 Issue 2 Sabin Vaccine Institute 2010 Albert B. Sabin Gold Medal Awarded to Dr. John D. Clemens During a ceremony at The George Washington University City View Room, Dr. John D. Clemens, Director General of the International Vaccine Institute in Seoul, Korea, received the 2010 Albert B. Sabin Gold Medal Award for his contributions to reducing suffering and promoting peace Dr. Steven Knapp, The George Washington through the development, University President; Dr. John D. Clemens; evaluation, and distribution of and Dr. Peter Hotez, Sabin President and Michael W. Marine, former The George Washington University vaccines. Distinguished Research Professor US Ambassador to the Socialist Republic of Dr. Clemens led the first efficacy trial of an oral vaccine against Vietnam, was appointed as cholera, and conducted additional research on a measles vaccine as the new chief executive a research scientist at the International Center for Diarrhoeal Disease officer of the Sabin Research, Bangladesh during the 1980s. IVI scientists transferred Vaccine Institute on April the technology for the cholera vaccine to Shantha Biotechics of 28, announced Sabin's Hyderabad, India, and in 2009, Shanchol™ was licensed for Chairman of the Board of development. Trustees, Mort Hyman and President Dr. Peter Hotez. Through his stewardship at the International Vaccine Institute in Seoul, Korea where he has served as Director General since 1999, Marine joined Sabin's Dr. Clemens has engaged in vaccine diplomacy in many areas, leadership team in including the Democratic People's Republic of Korea Program, which December 2009 after aims to reduce the disease burden of Hib and Japanese encephalitis serving eight months on in North Korean children by providing technical assistance in the Joint Action Committee laboratory diagnosis and surveillance of these diseases and in the of the Global Network for introduction of vaccines to prevent them. -

As Early As 1946, a Year Before Independence, the Government Of
Munich Personal RePEc Archive Gaining Technical Know-How in an Unequal World: Penicillin Manufacture in Nehru’s India Tyabji, Nasir April 2004 Online at https://mpra.ub.uni-muenchen.de/84236/ MPRA Paper No. 84236, posted 30 Jan 2018 04:51 UTC Gaining Technical Know-How in an Unequal World: Penicillin Manufacture in Nehru's India Nasir Tyabji I. Introduction For the first of the Jawaharlal Nehru Memorial lectures held at New Delhi in 1967, P.M.S. Blackett chose the theme of “Science and Technology in an Unequal World.”1 This was an apt choice of topic. For on the one hand, throughout his active public life, Jawaharlal Nehru had been convinced that the key to initiating a comprehensive process of development lay in the application of the results of scientific and technological enquiry to the problems confronting Indian society.2 Equally, Nehru was aware that India’s own scientific and technological base could not provide more than a small fraction of the effort required to provide the solutions to these problems.3 As the Prime Minister of Independent India, he was thus confronted with the task of identifying, and then accessing, foreign sources of technology. This was a task which raised complex issues, quite distinct from the technical problems of the “transfer of technology” from a foreign source to a local recipient.4 While Nehru’s experience as a nationalist politician might have provided him with an understanding of the power relations underlying the ground realities of international relations, the practical issues that might arise in the course of negotiating technology acquisitions could not be foreseen. -

Smallpox Vaccine and Vaccination in the Intensified Smallpox Eradication Programme
CHAPTER 11 SMALLPOX VACCINE AND VACCINATION IN THE INTENSIFIED SMALLPOX ERADICATION PROGRAMME Contents Page Introduction 540 Vaccine requirements for the Intensified Smallpox Eradica- tion Programme 541 Providing the finance 541 Shortfalls in vaccines-quality and quantity 542 WHO survey of vaccine producers 543 Methods of vaccine production 543 Strains of vaccinia virus 545 Number of doses per container 545 Initial potency 546 Heat stability 546 Bacterial count 547 Establishment of the WHO reference centres for smallpox vaccine 547 Development of improved vaccines 548 Meeting of experts (March 1968) 549 Production of freeze-dried vaccine 551 Continuing quality control of smallpox vaccine 554 Testing samples of vaccine 554 Visits by consultants 555 Reference vaccine 555 Seed lots of vaccine 556 Evaluation of testing methods 557 Improvements in vaccine quality 559 Vaccine production in endemic countries 563 Donations of vaccine and their distribution 564 New vaccination techniques 567 The bifurcated needle 567 Jet injectors 573 Modification to vaccination procedures 580 Preparation of skin 580 Neonatal vaccination 580 539 5 40 SMALLPOX AND ITS ERADICATION Page The search for new vaccines 580 Selection of vaccinia virus strains of low patho- genicity 581 Attenuated strains 583 Inactivated vaccines 587 Production of vaccine in eggs and tissue culture 588 Silicone ointment vaccine 590 Efficacy of vaccination 590 INTRODUCTION In May 1980 the Thirty-third World Health Assembly, after it had declared that Vaccination against smallpox had been smallpox had been eradicated throughout the practised in virtually every country of the world, recommended that smallpox vacci- world, and in many on a large scale, when the nation should be discontinued, except for in- Intensified Smallpox Eradication Programme vestigators at special risk . -

(GIN) Global Immunization News
Global Immunization News (GIN) Global Immunization News (GIN) May 2019 In this issue News You can click on the article 72nd World Health Assembly Special Report you are interested in and ac- Hayatee Hasan, WHO Headquarters cess it directly! This year’s World Health Assembly was held from 20 News to 28 May 2019 at the Palais des Nations in Geneva, • Immunization Agenda 2030 2 Switzerland and was attended by nearly 4000 delegates • Advancing digital health in 2 from WHO’s 194 Member States and partner organiza- Bangladesh through electron- ic immunization registration tions. The general theme of this year’s World Health • The world’s first malaria 3 Assembly (WHA) was "Universal health coverage: leav- vaccine highlighted at ing no-one behind”. #WHA72 The Assembly Hall at the Palais des Nations 3 in Geneva, Switzerland. • Malaria vaccine pilot Key highlights included: launched in Ghana • WHO announced the appointment of four new goodwill ambassadors from the fields of sports, • Over 100 000 people sick 3 with measles in 14 months: politics and community mobilization to promote healthier lives, stronger health workforces and with measles cases at an improved mental health globally. The new ambassadors are: alarming level in the Europe- 1. Alisson Becker, goalkeeper of the Brazilian national and Liverpool football teams; an Region, WHO scales up 2. Dr Natália Loewe Becker, medical doctor and health advocate from Brazil, as WHO response Goodwill Ambassadors for Health Promotion; • WHO adapts ebola vaccina- 4 tion strategy in the Demo- 3. Cynthia Germanotta, President of Born This Way Foundation, which was co-founded cratic Republic of the Congo with her daughter Lady Gaga, as WHO Goodwill Ambassador for Mental Health; and to account for insecurity and 4.