Smallpox Vaccine and Vaccination in the Intensified Smallpox Eradication Programme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modernization This Ispublic Health: Acanadianhistory
Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History CHAPTER 3 printable version of this chapter Modernization and Growth . 3 .1 Maternal and Child Health . 3 .2 Public Health Nurses . 3 .4 Full-Time Health Units . 3 .5 Services for Indigenous Communities . 3 .7 Venereal Disease . 3 .8 Charitable Organizations . 3 .9 Lapses in Oversight: Smallpox and Typhoid . 3 .11 Toronto’s School of Hygiene and Connaught Laboratories . 3 .14 Poliomyelitis . 3 .15 Depression and the End of Expansion . 3 .16 Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History 3.1 CHAPTER 3 Dominion Council of Health Modernization The .Dominion .Council .of .Health . was .chaired .by .the .federal .Deputy . Minister .of .Health .and .made .up .of . and Growth the .chief .provincial .officers .of .health . and .representatives .of .urban .and . A .new .public .health .order .emerged .in .the .aftermath .of .World .War .I, .represented .at .the . rural .women, .labour, .agriculture .and . universities, .the .latter .representing . international .level .by .the .development .of .the .Health .Organization .of .the .League .of .Nations . academic .and .scientific .expertise .in . In .Canada, .this .new .order .was .symbolized .by .the .Dominion .Council .of .Health .(DCH), .which . medicine, .public .health .and .laboratory . was .created .to .develop .policies .and .advise .the .new .federal .Department .of .Health . .Initially, . research . .The .Council .provided .a . the .Department .was .primarily .focused .on .collecting .and .distributing .information, .with .some . twice-yearly .forum .to .openly .discuss, . lesser .effort .to .develop .federal .laboratory .research .capacity . compare .and .co-ordinate .strategies .on . the .major .public .health .concerns .of .the . -

Vaccines and Autism: What You Should Know | Vaccine Education
Q A Vaccines and Autism: What you should know Volume& 1 Summer 2008 Some parents of children with autism are concerned that vaccines are the cause. Their concerns center on three areas: the combination measles-mumps-rubella (MMR) vaccine; thimerosal, a mercury-containing preservative previously contained in several vaccines; and the notion that babies receive too many vaccines too soon. Q. What are the symptoms of autism? Q. Does the MMR vaccine cause autism? A. Symptoms of autism, which typically appear during the A. No. In 1998, a British researcher named Andrew Wakefi eld fi rst few years of life, include diffi culties with behavior, social raised the notion that the MMR vaccine might cause autism. skills and communication. Specifi cally, children with autism In the medical journal The Lancet, he reported the stories of may have diffi culty interacting socially with parents, siblings eight children who developed autism and intestinal problems and other people; have diffi culty with transitions and need soon after receiving the MMR vaccine. To determine whether routine; engage in repetitive behaviors such as hand fl apping Wakefi eld’s suspicion was correct, researchers performed or rocking; display a preoccupation with activities or toys; a series of studies comparing hundreds of thousands of and suffer a heightened sensitivity to noise and sounds. children who had received the MMR vaccine with hundreds Autism spectrum disorders vary in the type and severity of of thousands who had never received the vaccine. They found the symptoms they cause, so two children with autism may that the risk of autism was the same in both groups. -

The Spanish Flu and Canadian Influenza Vaccine Initiatives
DefiningMomentsCanada.ca THE SPANISH FLU AND CANADIAN INFLUENZA VACCINE INITIATIVES Christopher J. Rutty, Ph.D. significant but sometimes overlooked element in the history of the Spanish Flu pandemic of 1918-19 A was the experimental production, distribution and wide use of influenza vaccines. Since the vaccines were based on an erroneous view that influenza was caused by a bacteria (the influenza virus would not be isolated until 1933) such vaccines were ineffective and thus of little importance to the course of the pandemic from a medical or public health perspective. However, the story of the vaccines produced to prevent pandemic influenza, particularly in Canada, reveals much about the application of uncertain knowledge in the face of an unprecedented public health emergency. It also reveals the changing state of Canadian biotechnology capacity at the end of World War I. Connaught Antitoxin Laboratories of the University of Toronto led the most significant initiative to produce an influenza vaccine.1 Connaught’s flu vaccine initiative coincided with a significant production effort by the Ontario Provincial Laboratories, along with various smaller scale and more local efforts, particularly in Kingston and Winnipeg.2 Canadian vaccine efforts were also linked to emergency vaccine preparation in New York City, Boston, and at the Mayo Clinic in Minnesota.3 Influenza vaccines were similarly prepared in other countries in the face of the global pandemic. In 1919, Dr. John G. FitzGerald, Director of Connaught Antitoxin Laboratories, began his summary of Connaught’s influenza vaccine work by observing: “almost coincident with the end of the war a great emergency arose in which the laboratories were provided with an opportunity of doing public service work of a national character.”4 Connaught had been established in May, 1914, as the Antitoxin Laboratory in the Department of Hygiene, 1 Online resources about the history of Connaught Laboratories include: http://connaught.research.utoronto.ca/history/; http:// thelegacyproject.ca 2 J.W.S. -

Correspondence, Research Notes and Papers, Articles
MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology Correspondence, research notes and papers, articles, speeches, travel journals, drawings, and sketches, photographs, clippings, and other memorabilia, awards and prizes. Includes some papers from his widow Henrietta Banting (d. 1976). 1908-1976. Extent: 63 boxes (approx. 8 metres) Part of the collection was deposited in the Library in 1957 by the “Committee concerned with the Banting Memorabilia”, which had been set up after the death of Banting in 1941. These materials included papers from Banting’s office. At the same time the books found in his office (largely scientific and medical texts and journals) were also deposited in the University Library. These now form a separate collection in the Thomas Fisher Rare Book Library. The remainder of the collection was bequeathed to the Thomas Fisher Rare Book Library by Banting’s widow, Dr. Henrietta Banting, in 1976. This part of the collection included materials collected by Henrietta Banting for her projected biography of F.G. Banting, as well as correspondence and memorabilia relating to her won career. Researchers who wish to publish extensively from previously unpublished material from this collection should discuss the question of literary rights with: Mrs. Nancy Banting 12420 Blackstock Street Maple Ridge, British Columbia V2X 5N6 (1989) Indicates a letter of application addressed to the Director, Thomas Fisher Rare Book Library, is needed due to fragility of originals or confidential nature of documents. 1 MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology 1891 FGB born in Alliston, Ont. To Margaret (Grant) and William Thompson Banting. -

COVID-19 Vaccinations with an Update from 12/16/2020
COVID-19 Vaccinations with an Update from 12/16/2020 Michael A. Kaufmann, MD, FACEP, FAEMS State EMS Medical Director Indiana Department of Homeland Security Hot Off The Press • 12/11/2020 • Vaccine advisers to the US Food and Drug Administration voted Thursday to recommend the agency grant emergency use authorization to Pfizer and BioNTech's coronavirus vaccine. • Seventeen members of the Vaccines and Related Biological Products Advisory Committee voted yes, four voted no and one abstained. • "The question is never when you know everything. It's when you know enough and I think we know enough now to say that this appears to be our way out of this awful, awful mess," Dr. Paul Offit, director of the Vaccine Education Center at Children's Hospital of Philadelphia and a member of the committee, told CNN's Wolf Blitzer after the vote. • "That's why I voted yes." FDA Concerns • Several committee members expressed concern about reports of allergic reactions in two people who were vaccinated in Britain, which authorized Pfizer's vaccine ahead of the US. • FDA staff said that, as with any vaccines, paperwork would accompany the Pfizer vaccine to warn against administering it to anyone with a history of severe allergic reactions to vaccines or allergies to any of the ingredients of the vaccine. • The FDA will now decide whether to accept the recommendation, but has signaled that it will issue the EUA for the vaccine. • ACIP has a meeting scheduled for Friday, and expects to vote during a meeting scheduled for Sunday. • Operation Warp Speed officials say they will start shipping the vaccine within 24 hours of FDA authorization. -

Humanized Mice for Live-Attenuated Vaccine Research: from Unmet Potential to New Promises
Review Humanized Mice for Live-Attenuated Vaccine Research: From Unmet Potential to New Promises Aoife K. O’Connell and Florian Douam * Department of Microbiology, National Emerging Infectious Diseases Laboratories, Boston University School of Medicine, Boston, MA 02118, USA; [email protected] * Correspondence: [email protected] Received: 21 December 2019; Accepted: 13 January 2020; Published: 21 January 2020 Abstract: Live-attenuated vaccines (LAV) represent one of the most important medical innovations in human history. In the past three centuries, LAV have saved hundreds of millions of lives, and will continue to do so for many decades to come. Interestingly, the most successful LAVs, such as the smallpox vaccine, the measles vaccine, and the yellow fever vaccine, have been isolated and/or developed in a purely empirical manner without any understanding of the immunological mechanisms they trigger. Today, the mechanisms governing potent LAV immunogenicity and long-term induced protective immunity continue to be elusive, and therefore hamper the rational design of innovative vaccine strategies. A serious roadblock to understanding LAV-induced immunity has been the lack of suitable and cost-effective animal models that can accurately mimic human immune responses. In the last two decades, human-immune system mice (HIS mice), i.e., mice engrafted with components of the human immune system, have been instrumental in investigating the life-cycle and immune responses to multiple human-tropic pathogens. However, their use in LAV research has remained limited. Here, we discuss the strong potential of LAVs as tools to enhance our understanding of human immunity and review the past, current and future contributions of HIS mice to this endeavor. -

Myocarditis After SARS-Cov-2 Vaccination: True, True, and … Related? Sean T
Myocarditis After SARS-CoV-2 Vaccination: True, True, and … Related? Sean T. O’Leary, MD, MPH,a,b Yvonne A. Maldonado, MDc In this month’s issue of Pediatrics, compiled cases through personal Marshall et al report a case series communication rather than through describing seven 14- to 19-year-old a systematic surveillance system. male individuals who developed Clearly, this approach could symptomatic myocarditis after the introduce reporting bias, and a case second dose of the Pfizer-BioNTech series cannot establish causality. coronavirus disease 2019 (COVID- Another critical point to consider is 19) vaccine.1 The authors report that that the reported cases mirror the the symptoms began between 2 and seasonal prevalence, sex, and age 4 days after the second dose and that profile of background cases of aAdult and Child Consortium for Health Outcomes all 7 patients experienced rapid myocarditis, thereby complicating an Research and Delivery Science, Anschutz Medical Campus, resolution of symptoms. This case assessment of a potential association University of Colorado and Children’s Hospital Colorado, Aurora, Colorado, bDepartment of Pediatrics, Anschutz series is published in the context of with SARS-CoV-2 vaccines. The Medical Campus, University of Colorado, Aurora, Colorado, other media reports of myocarditis authors themselves note in their cDepartment of Pediatrics, School of Medicine, Stanford in young adults, mostly male, from discussion the high background rate University, Stanford, California 2 the US military and from Israel as of myocarditis in adolescent and Opinions expressed in these commentaries are those of well as a recent increase in reports young adult male individuals,4 the authors and not necessarily those of the American Academy of Pediatrics or its Committees. -
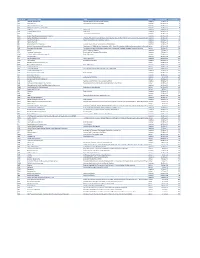
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

Diseases Subject to the International Health Regulations
Diseases Subject to the International Health Regulations Cholera, yellow fever, and plague cases and deaths reported in the Region of the Americas up to 15 October 1980 Country and Yellow fever administrative Cholera Plague subdivision Cases Cases Deaths Cases BOLIVIA - 46 39 15 Cochabamba - 12 8 - La Paz - 32 30 15 Santa Cruz - 1 1 - Tarija - 1 - BRAZIL - 25 22 69 Ceará - - - 62 Goiás - 20 19 Maranhao - 4 2 - Pernambuco - - - 7 Rondónia - 1 1 - CANADA 3 - - - Quebec 1 - - Saskatchewan 2 - - COLOMBIA - 7 7 - Cesar - 1 1 - Guaviare - 1 1 - Meta - 1 1 - Norte de Santander - 1 1 - Putumayo - 3 3 - ECUADOR - 2 - Napo - 2 PERU - 24 19 - Ayacucho - 8 7 - Junín - 7 4 - San Martín - 7 7 - ...- 2 1 - UNITED STATES 8 - - 13 California 6 - 2 Maryland 1 - Nevada - - - 2 New Mexico - - - 9 Pennsylvania 1 - - VENEZUELA - 1 1 - Mérida - 1 1 -None. ... Data not available. I Accidental Smallpox Vaccination in Venezuela On 31 July 1980 a report was received that the previous vaccine (lot No. 48 produced by the National Institute of day a 10-month old child weighing 10 kg, who had been Health), rehydrated in the diluent of the Merieux Lab- taken for measles vaccination in Barquisimeto, Lara oratory measles vaccine. In a way, the accident provided State, had accidentally received in the left arm a sub- an opportunity for reinforcing the principle that those in cutaneous injection of 25 doses of freeze-dried smallpox charge of programs should supervise the immunizations 5 more closely. It also provided an example of what can The fact that smallpox vaccine was administered at all happen, and the state epidemiologists who were attend- prompts yet another important comment. -

Chronological Introduction of Types of Vaccine Products That Are Still Licensed in the United States
Appendix 2.3 CHRONOLOGICAL INTRODUCTION OF TYPES OF VACCINE PRODUCTS THAT ARE STILL LICENSED IN THE UNITED STATES The year of introduction of each of 49 of the 51 shown in table 2.3B, American pharmaceutical com- types of vaccine prducts currently licensed in the panies were issued 37 (89 percent) of the original United States, alolng with the manufacturing estab- licenses for these 42 products. New or improved lishment with the oldest license still in effect for each types of products that are currently licensed have product, is shown in table 2.3 A.] For 42 (86 percent) been introduced at a fairly consistent rate of three to of these 49 products, the establishment that received seven products per each 5-year interval since 1940.2 the original product license still holds this license. As Ten of the currently licensed products were licensed before 1940. Table 2.3A—Chronological Introduction of Types of Vaccine Products Still Licensed in the United States Year Type of vaccine product Establishment with oldest product license still in effecta 1903 Dlphtherla antitoxin Massachusetts Public Health Biologic Laboratories (191 7) 1907 Tetanus antitoxin Parke. Davis and Company (191 5) 1914 Pertussis vaccine Lederle Laboratories Typhoid vaccine Massachusetts Public Health Biologic Laboratories (191 7) Rabies vaccine . Eli Lilly and Company* 1917 Cholera vaccine Eli Lilly and Company* 1926 Diphtheria toxoid Parke, Davis and Company (1927) 1933 Staphylococcus toxoid Lederle Laboratories* Tetanus toxoid Merck Sharp and Dohme 1941 Typhus vaccine Eli Lilly and Company 1942 Plague vaccine .., . Cutter Laboratories* Rocky Mountain Spotted Fever vaccine. Lederle Laboratories* 1945 InfIuenza virus vaccine Lederle Laboratories Merck Sharp and Dohme Parke, Davis and Company * 1946 Parke, Davis and Company ● 1947 Parke, Davis and Company (1949) 1948 Parke, Davis and Company (1952) Parke. -

Key Messages and Answers About Vaccine Safety MANUAL for HEALTH WORKERS
Key Messages and Answers about Vaccine Safety MANUAL FOR HEALTH WORKERS Key Messages and Answers about Vaccine Safety MANUAL FOR HEALTH WORKERS Washington, D.C., 2021 Key Messages and Answers about Vaccine Safety. Manual for Health Care Workers PAHO/FPL/IM/COVID-19/21-0027 © Pan American Health Organization, 2021 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO license (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this license, this work may be copied, redistributed, and adapted for non-commercial purposes, provided the new work is issued using the same or equivalent Creative Commons license and it is appropriately cited. In any use of this work, there should be no suggestion that the Pan American Health Organization (PAHO) endorses any specific organization, product, or service. Use of the PAHO logo is not permitted. All reasonable precautions have been taken by PAHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall PAHO be liable for damages arising from its use. ii KEY MESSAGES AND ANSWERS ABOUT VACCINE SAFETY Contents Acknowledgements VI Introduction 1 History of vaccines up to the present 2 Chapter 1. Immunization schedules in the regular program 4 1.1 Key messages 5 1.2 Questions and answers -

Vaccine Lessons from the Early 1800S: the Boon of Jenner and Covid-19
Vaccine lessons from the early 1800s: The Boon of Jenner and Covid-19 Item Type Blog Authors Takemoto, Hanna Publication Date 2021-05-10 Abstract COVID-19 vaccines allowed the world to open back up after months of quarantine, social distancing, and masking. This post traces the history of vaccines and the hesitancy to vaccinate from Edward Jenner's discovery of the smallpox vaccine. The post... Keywords Dissertation; Boon of Jenner; Vaccines; Jenner, Edward, 1749-1823; Smallpox; COVID-19 (Disease); University of Maryland, Baltimore. School of Medicine; Smallpox; COVID-19; Vaccines Download date 01/10/2021 16:54:33 Link to Item http://hdl.handle.net/10713/16241 Vaccine lessons from the early 1800s: The Boon of Jenner and Covid-19 Posted May 10, 2021 Written by Hanna Takemoto, Spring 2021 HSHSL Intern Hanna Takemoto is a new graduate of the MLIS program at the University of Maryland, College Park. She recently completed an internship at the HSHSL where she worked on a collection of 19th century School of Medicine dissertations. As the world continues to grapple with the relentless coronavirus, vaccines remain the most promising tool in our arsenal. In fact, we are in the midst of what can be described as the most ambitious vaccination effort in human history. The unprecedented speed of the Covid-19 vaccine development has been a source of pride and hope, but also anxiety. According to the latest estimates by the U.S. Census Bureau, 15% of Americans do not plan on getting vaccinated against Covid-19. When we consider the successful vaccination campaigns of the 20th century that eradicated polio, tuberculosis, and measles, vaccine hesitancy may seem like a modern phenomenon fuelled by the online spread of misinformation.