Chronological Introduction of Types of Vaccine Products That Are Still Licensed in the United States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modernization This Ispublic Health: Acanadianhistory
Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History CHAPTER 3 printable version of this chapter Modernization and Growth . 3 .1 Maternal and Child Health . 3 .2 Public Health Nurses . 3 .4 Full-Time Health Units . 3 .5 Services for Indigenous Communities . 3 .7 Venereal Disease . 3 .8 Charitable Organizations . 3 .9 Lapses in Oversight: Smallpox and Typhoid . 3 .11 Toronto’s School of Hygiene and Connaught Laboratories . 3 .14 Poliomyelitis . 3 .15 Depression and the End of Expansion . 3 .16 Table of Contents Endnotes – Glossary Credits Profiles Contact CPHA 1920This is Public Health: A Canadian1929 History 3.1 CHAPTER 3 Dominion Council of Health Modernization The .Dominion .Council .of .Health . was .chaired .by .the .federal .Deputy . Minister .of .Health .and .made .up .of . and Growth the .chief .provincial .officers .of .health . and .representatives .of .urban .and . A .new .public .health .order .emerged .in .the .aftermath .of .World .War .I, .represented .at .the . rural .women, .labour, .agriculture .and . universities, .the .latter .representing . international .level .by .the .development .of .the .Health .Organization .of .the .League .of .Nations . academic .and .scientific .expertise .in . In .Canada, .this .new .order .was .symbolized .by .the .Dominion .Council .of .Health .(DCH), .which . medicine, .public .health .and .laboratory . was .created .to .develop .policies .and .advise .the .new .federal .Department .of .Health . .Initially, . research . .The .Council .provided .a . the .Department .was .primarily .focused .on .collecting .and .distributing .information, .with .some . twice-yearly .forum .to .openly .discuss, . lesser .effort .to .develop .federal .laboratory .research .capacity . compare .and .co-ordinate .strategies .on . the .major .public .health .concerns .of .the . -

Polio Vaccine Safety Paul Meier's Role in the Discovery And
www.barkerstats.com Polio Vaccine the cutter incident October 1, 2020 Downloaded from www.barkerstats.com/PDFs/Meier/Meier-Cutter-Incident-History.pdf Polio Vaccine Safety Paul Meier’s Role in the Discovery and Evaluation of The Cutter Incident Professor Chris Barker, Ph.D. Adjunct Associate Professor of Biostatistics, UIC SPH. www.barkerstats.com Page 1 of 11 www.barkerstats.com Polio Vaccine the cutter incident October 1, 2020 Contents Background .................................................................................................................................................................................................................... 3 Brief Polio Vaccine Overview ......................................................................................................................................................................................... 3 Polio Vaccine Manufacturing Data Suppression ............................................................................................................................................................ 4 Biological Disaster? How Serious was the vaccine manufacturing problem? ............................................................................................................... 5 Near Elimination of Vaccine development by lawsuits ................................................................................................................................................. 6 Meier’s evaluation of the safety recommendations following The Cutter Incident .................................................................................................... -

Our Global Initiative
2021 OUR GLOBAL INITIATIVE AN ASSESSMENT OF COVID-19 & THE FAILED GLOBAL HEALTH POLICIES OF THE WHO OUR GLOBAL INITIATIVE In early 2020, Australia, and indeed the world, collapsed into a lockdown prompted by the World Health Organisation in its designation of Covid 19 as a pandemic. What began as “2 weeks to flatten the curve” quickly morphed into a year of intermittent lockdowns, a loss of freedoms and liberties, the decimations of SME’s, irrational restrictions, unadulterated government surveillance, an endless state of emergencies, the rise of the police state a totalitarian grand stride towards medical fascism and a technocratic dictatorship as well as the end of civil society as we know it. What happened? How did we get here? Where are we going? What does this world of the “New Normal” hold for not only Australia, but the human civilisation? How do we navigate this reality and not only survive but thrive? The Global Health Organisation, in collaboration with the Australian Patriots Alliance, the World Solutions Foundation, the QuantumPrism Foundation, Ikighais Pty Ltd and other concerned institutions (the Initiative) have commissioned this report in effort to gain an insight into what is going on; the why, they how and the WHO! Further, the report will offer solutions to current concerns, as well as ways for interested parties to position themselves to thrive and win in the new society bound to emerge from the ashes of this dying world. 2 TIPS TO HELP READ THIS REPORT This report contains a lot of information and we appreciate SUMMARY POINTS that time is scarce. -

The Spanish Flu and Canadian Influenza Vaccine Initiatives
DefiningMomentsCanada.ca THE SPANISH FLU AND CANADIAN INFLUENZA VACCINE INITIATIVES Christopher J. Rutty, Ph.D. significant but sometimes overlooked element in the history of the Spanish Flu pandemic of 1918-19 A was the experimental production, distribution and wide use of influenza vaccines. Since the vaccines were based on an erroneous view that influenza was caused by a bacteria (the influenza virus would not be isolated until 1933) such vaccines were ineffective and thus of little importance to the course of the pandemic from a medical or public health perspective. However, the story of the vaccines produced to prevent pandemic influenza, particularly in Canada, reveals much about the application of uncertain knowledge in the face of an unprecedented public health emergency. It also reveals the changing state of Canadian biotechnology capacity at the end of World War I. Connaught Antitoxin Laboratories of the University of Toronto led the most significant initiative to produce an influenza vaccine.1 Connaught’s flu vaccine initiative coincided with a significant production effort by the Ontario Provincial Laboratories, along with various smaller scale and more local efforts, particularly in Kingston and Winnipeg.2 Canadian vaccine efforts were also linked to emergency vaccine preparation in New York City, Boston, and at the Mayo Clinic in Minnesota.3 Influenza vaccines were similarly prepared in other countries in the face of the global pandemic. In 1919, Dr. John G. FitzGerald, Director of Connaught Antitoxin Laboratories, began his summary of Connaught’s influenza vaccine work by observing: “almost coincident with the end of the war a great emergency arose in which the laboratories were provided with an opportunity of doing public service work of a national character.”4 Connaught had been established in May, 1914, as the Antitoxin Laboratory in the Department of Hygiene, 1 Online resources about the history of Connaught Laboratories include: http://connaught.research.utoronto.ca/history/; http:// thelegacyproject.ca 2 J.W.S. -

Correspondence, Research Notes and Papers, Articles
MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology Correspondence, research notes and papers, articles, speeches, travel journals, drawings, and sketches, photographs, clippings, and other memorabilia, awards and prizes. Includes some papers from his widow Henrietta Banting (d. 1976). 1908-1976. Extent: 63 boxes (approx. 8 metres) Part of the collection was deposited in the Library in 1957 by the “Committee concerned with the Banting Memorabilia”, which had been set up after the death of Banting in 1941. These materials included papers from Banting’s office. At the same time the books found in his office (largely scientific and medical texts and journals) were also deposited in the University Library. These now form a separate collection in the Thomas Fisher Rare Book Library. The remainder of the collection was bequeathed to the Thomas Fisher Rare Book Library by Banting’s widow, Dr. Henrietta Banting, in 1976. This part of the collection included materials collected by Henrietta Banting for her projected biography of F.G. Banting, as well as correspondence and memorabilia relating to her won career. Researchers who wish to publish extensively from previously unpublished material from this collection should discuss the question of literary rights with: Mrs. Nancy Banting 12420 Blackstock Street Maple Ridge, British Columbia V2X 5N6 (1989) Indicates a letter of application addressed to the Director, Thomas Fisher Rare Book Library, is needed due to fragility of originals or confidential nature of documents. 1 MS BANTING (FREDERICK GRANT, SIR) PAPERS COLL Papers 76 Chronology 1891 FGB born in Alliston, Ont. To Margaret (Grant) and William Thompson Banting. -
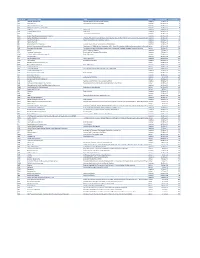
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

As Early As 1946, a Year Before Independence, the Government Of
Munich Personal RePEc Archive Gaining Technical Know-How in an Unequal World: Penicillin Manufacture in Nehru’s India Tyabji, Nasir April 2004 Online at https://mpra.ub.uni-muenchen.de/84236/ MPRA Paper No. 84236, posted 30 Jan 2018 04:51 UTC Gaining Technical Know-How in an Unequal World: Penicillin Manufacture in Nehru's India Nasir Tyabji I. Introduction For the first of the Jawaharlal Nehru Memorial lectures held at New Delhi in 1967, P.M.S. Blackett chose the theme of “Science and Technology in an Unequal World.”1 This was an apt choice of topic. For on the one hand, throughout his active public life, Jawaharlal Nehru had been convinced that the key to initiating a comprehensive process of development lay in the application of the results of scientific and technological enquiry to the problems confronting Indian society.2 Equally, Nehru was aware that India’s own scientific and technological base could not provide more than a small fraction of the effort required to provide the solutions to these problems.3 As the Prime Minister of Independent India, he was thus confronted with the task of identifying, and then accessing, foreign sources of technology. This was a task which raised complex issues, quite distinct from the technical problems of the “transfer of technology” from a foreign source to a local recipient.4 While Nehru’s experience as a nationalist politician might have provided him with an understanding of the power relations underlying the ground realities of international relations, the practical issues that might arise in the course of negotiating technology acquisitions could not be foreseen. -

Smallpox Vaccine and Vaccination in the Intensified Smallpox Eradication Programme
CHAPTER 11 SMALLPOX VACCINE AND VACCINATION IN THE INTENSIFIED SMALLPOX ERADICATION PROGRAMME Contents Page Introduction 540 Vaccine requirements for the Intensified Smallpox Eradica- tion Programme 541 Providing the finance 541 Shortfalls in vaccines-quality and quantity 542 WHO survey of vaccine producers 543 Methods of vaccine production 543 Strains of vaccinia virus 545 Number of doses per container 545 Initial potency 546 Heat stability 546 Bacterial count 547 Establishment of the WHO reference centres for smallpox vaccine 547 Development of improved vaccines 548 Meeting of experts (March 1968) 549 Production of freeze-dried vaccine 551 Continuing quality control of smallpox vaccine 554 Testing samples of vaccine 554 Visits by consultants 555 Reference vaccine 555 Seed lots of vaccine 556 Evaluation of testing methods 557 Improvements in vaccine quality 559 Vaccine production in endemic countries 563 Donations of vaccine and their distribution 564 New vaccination techniques 567 The bifurcated needle 567 Jet injectors 573 Modification to vaccination procedures 580 Preparation of skin 580 Neonatal vaccination 580 539 5 40 SMALLPOX AND ITS ERADICATION Page The search for new vaccines 580 Selection of vaccinia virus strains of low patho- genicity 581 Attenuated strains 583 Inactivated vaccines 587 Production of vaccine in eggs and tissue culture 588 Silicone ointment vaccine 590 Efficacy of vaccination 590 INTRODUCTION In May 1980 the Thirty-third World Health Assembly, after it had declared that Vaccination against smallpox had been smallpox had been eradicated throughout the practised in virtually every country of the world, recommended that smallpox vacci- world, and in many on a large scale, when the nation should be discontinued, except for in- Intensified Smallpox Eradication Programme vestigators at special risk . -

The Academic Model for the Prevention and Treatment of HIV
C A S E S I N G L O B A L H E A L T H D ELIVERY GHD-045 JULY 2021 Building the Supply Chain for COVID-19 Vaccines Mid-April 2020 saw a rapid escalation of the COVID-19 pandemic. In the four months after December 2019, when the novel coronavirus that causes COVID-19 was first detected in Wuhan, China, the virus had infected several million people globally, with more than a hundred thousand confirmed deaths (see Exhibit 1 for daily confirmed deaths by country). China and Italy experienced major outbreaks early and saw hospitals flooded with COVID-19 patients, causing major shortages of vital intensive-care materials. To forestall the overburdening of health care resources, more than a dozen major countries imposed strict lockdowns to slow the spread of the disease, or “flatten the curve” (see Exhibit 2 for a map of government responses). Government lockdowns disrupted supply and demand in vital industries including retail, tourism, manufacturing, and services, crippling the global economy. As the massive scale of the crisis became apparent, financial markets began to collapse during February, leading some experts to warn of a potential next Great Depression and governments to announce unprecedented rescue packages to contain the destructive economic impact of the crisis. As governments navigated trade-offs between economic and public health outcomes, a global race had begun for the rapid discovery, production, and distribution of a safe and effective vaccine. The organization of supply chains to manufacture, distribute, and administer a vaccine to a sufficient portion of the 7.6 billion world population to contain the disease, a concept termed “herd immunity,” posed significant challenges. -

Robert J. Desalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944
Robert J. DeSalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944 - 1990 Collection #107 Abstract Robert J. DeSalvo’s research focused on business combinations (acquisitions, mergers, and joint ventures in the cosmetic and pharmaceutical industries. This topic was the basis for his master’s thesis in pharmacy administration at the University of Pittsburgh and continued as a life-long interest. This collection consists of two series of notebooks that Dr. DeSalvo developed to record relevant business combinations. The first series records acquisitions, proposed acquisitions, mergers, and joint ventures for the period of 1944 –1990 in an alphabetical arrangement. The information on these entries is cumulative so that the history of an organization is collected in one place. The second series of notebooks is arranged in chronological blocks. The information is arranged alphabetically by the name of the acquirer. The name of the acquired (merged), type of combination (acquisition, proposed acquisition, joint venture) and the date is also provided. The information is cross-referenced between the two series so that the researcher can approach the information by the name of the parent company or chronologically. Dr. DeSalvo used this resource for many of his publications as well as his master’s thesis. A copy of these publications and his thesis make up the remainder of the collection. Donor Gift of Barbara DeSalvo, 2000 Biography Robert James DeSalvo was born on July 20, 1933 in Toledo, OH. He died on January 23, 1993 in Cincinnati, OH. DeSalvo graduated from high school in Toledo and attended pharmacy school at the University of Toledo where he received his B.S. -
![[1] Vaccination Update Table of Contents: 1](https://docslib.b-cdn.net/cover/7215/1-vaccination-update-table-of-contents-1-3097215.webp)
[1] Vaccination Update Table of Contents: 1
EYE ON THE MARKET • MICHAEL CEMBALEST • J.P. MORGAN Last updated 9/23/2021 [1] Vaccination update Table of Contents: 1. US vaccination overview ................................................................................................................................2 2. Vaccination vs infection, mortality, hospitalization and 2020 Trump voting share ............................................3 3. “A pandemic of the unvaccinated” .................................................................................................................4 4. Population shares, vaccine efficacy and the amalgamation paradox ................................................................5 5. Vaccine efficacy tracker ..................................................................................................................................6 6. Vaccination vs previous COVID infection (acquired vs natural immunity) .........................................................7 7. Vaccine risk-benefit data ................................................................................................................................8 8. Variant prevalence by country ........................................................................................................................9 9. Delta variant facts and figures ........................................................................................................................9 10. Vaccine update by country and US state ...................................................................................................... -

Fifty Years of Insulin
24 Ju1ie 1971 S.-A. MEDIESE TYDSKRIF 815 Fifty Years of Insulin Emaciation, weakness, coma and death. The VlctlffiS were of sugar and other carbohydrates. He would then inject the children. The outcome was always the same. Only deliberate extract into a second dog who had diabetes. On 30 July 1921 starvation could slow the lethal course of the disease. Banting performed the experiment that con.firmed the existence This is a picture of juvenile diabetes in 1921. In that year, of insulin. He removed the pancreas of the dog Marjorie and an obscure Canadian physician, Frederick Banting, made then kept her alive and active for 76 days with injections of an medical history when he discovered the hormone insulin and extract made from the pancreas glands of other animals. He later provided its ability to control diabetes. Insulin, of course, finally sacrificed her on 14 October to check for possible has since saved millions of lives throughout the world. The internal damage from the extract. He found no evidence of year 197 I is the 50th anniversary of Banting's historic discovery. injury. The story of insulin actually began on the night of 30 October An impaired ability to utilize carbohydrates is, of course, 1920. Young Dr Banting, then a demonstrator in physiology the basic problem in the disease. Juvenile diabetics, especially, at the University of Western Ontario, arose at two in the have a severe impairment. morning to jot down procedures for an experiment that would Because of limited laboratory facilities at Western Ontario, eventually reveal the life-saving hormone.