Mycoflora Associated with the Goat's Hair and Sheep Wool in Taif, Saudi
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

43-09-405 and 407 Final Combine
Pak. J. Bot ., 44(6): 2093-2102, 2012. A NEW SPECIES OF TIAROSPORELLA AZADARICHTA AND NEW FUNGAL RECORDS ON AZADIRACHTA INDICA FROM PAKISTAN SYED QAISER ABBAS 1, TEHREEMA IFTIKHAR 1* , MUBASHIR NIAZ 1, IFTIKHAR ALI 1, NABILA IFTIKHAR 1 AND ALIA ABBAS 2 1Department of Botany, Government College University, Alama Iqbal Road, Faisalabad, Pakistan 2Department of Botany, Federal Urdu University, Karachi, Pakistan *Corresponding author’s e-mail: [email protected] Abstract A new species of Tiarosporella azadarichta has been described on Azadirachta indica and some new fungal records viz ., Diplozythiella bambusina , Ulocladium chartarum , Cladosporium nigrellum, Cladosporium oxysporum. Didymostilbe coffeae , Muellerella pygmaea, Lasiodiplodia paraphysaria, Monochaetinula terminalae, Trimmatostroma sp., and Epidermophyton floccosum are reported on Azadirachta indica for the first time from Pakistan. Introduction reason a detailed survey of the plant for fungi was under taken in this study. Azadirachta indica (as Azadirachta Juss, Melia azadirachta ) belong to the family Meliaceae. Neem is its Materials and Methods common name. It is important due to its commercial and medicinal value. Azadirachta indica is also an important Samples of Azadirachta indica were collected from plant for its potential of anti-fungal, anti-bacterial and the different areas of District Faisalabad and Gojra. The insecticidal activity. Decline of trees due to fungi are different areas were G.C. University. Faisalabad, increasing tremendously in Pakistan especially in Punjab Agriculture University Faisalabad, Gutwala forest (park) and Sindh (Javed et al ., 2004; Khanzada et al ., 2011; Fateh Faisalabad, Tandlianwala and Gojra City. Methods and et al., 2011; Rheman et al., 2011; Farooq et al., 2011). materials are the same as described Abbas et al ., (2010). -

An Unusual Haemoid Fungi: Ulocladium, As a Cause Of
Volume 2 Number 2 (June 2010) 95-97 CASE Report An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis Kaur R, Wadhwa A1,Gulati A2, Agrawal AK2 1Department of Microbiology. 2Department of Otorhinolaryngology, Maulana Azad Medical College, New Delhi, India. Received: April 2010, Accepted: May 2010. ABSTRACT Allergic fungal sinusitis (AFS) has been recognized as an important cause of chronic sinusitis commonly caused by Aspergillus spp. and various dematiaceous fungi like Bipolaris, Alternaria, Curvalaria, and etc. Ulocladium botrytis is a non pathogenic environmental dematiaceous fungi, which has been recently described as a human pathogen. Ulocladium has never been associated with allergic fungal sinusitis but it was identified as an etiological agent of AFS in a 35 year old immunocompetent female patient presenting with chronic nasal obstruction of several months duration to our hospital. The patient underwent FESS and the excised polyps revealed Ulocladium as the causative fungal agent. Keywords: Ulocladium, Phaeoid Fungi, Chronic Sinusitis. INTRODUCTION CASE REPORT Chronic allergic sinusitis is a common condition A 35 year old female patient presented to the ENT responsible for the development of nasal polyps, out patient department of the Lok Nayak Hospital, described as abnormal lesions that emanate from Delhi, with chronic nasal obstruction, excessive any portion of the nasal mucosa or Para nasal sneezing, nasal discharge and frontal headache since sinuses. They are commonly located in the middle several months. Nasal obstruction was of gradual meatus and ethmoid sinus and are present in onset, non progressive, more on the left side than 1-4% of the population (1). -
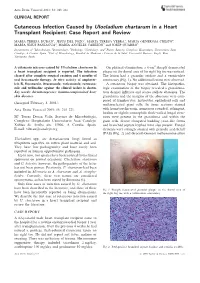
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Monograph on Dematiaceous Fungi
Monograph On Dematiaceous fungi A guide for description of dematiaceous fungi fungi of medical importance, diseases caused by them, diagnosis and treatment By Mohamed Refai and Heidy Abo El-Yazid Department of Microbiology, Faculty of Veterinary Medicine, Cairo University 2014 1 Preface The first time I saw cultures of dematiaceous fungi was in the laboratory of Prof. Seeliger in Bonn, 1962, when I attended a practical course on moulds for one week. Then I handled myself several cultures of black fungi, as contaminants in Mycology Laboratory of Prof. Rieth, 1963-1964, in Hamburg. When I visited Prof. DE Varies in Baarn, 1963. I was fascinated by the tremendous number of moulds in the Centraalbureau voor Schimmelcultures, Baarn, Netherlands. On the other hand, I was proud, that El-Sheikh Mahgoub, a Colleague from Sundan, wrote an internationally well-known book on mycetoma. I have never seen cases of dematiaceous fungal infections in Egypt, therefore, I was very happy, when I saw the collection of mycetoma cases reported in Egypt by the eminent Egyptian Mycologist, Prof. Dr Mohamed Taha, Zagazig University. To all these prominent mycologists I dedicate this monograph. Prof. Dr. Mohamed Refai, 1.5.2014 Heinz Seeliger Heinz Rieth Gerard de Vries, El-Sheikh Mahgoub Mohamed Taha 2 Contents 1. Introduction 4 2. 30. The genus Rhinocladiella 83 2. Description of dematiaceous 6 2. 31. The genus Scedosporium 86 fungi 2. 1. The genus Alternaria 6 2. 32. The genus Scytalidium 89 2.2. The genus Aurobasidium 11 2.33. The genus Stachybotrys 91 2.3. The genus Bipolaris 16 2. -

Recurrence of Stachybotrys Chartarum During Mycological And
Recurrence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed Caroline Lanier, Veronique Andre, Virginie Seguin, Natacha Heutte, Anne El Kaddoumi, Valérie Bouchart, Rachel Picquet, David Garon To cite this version: Caroline Lanier, Veronique Andre, Virginie Seguin, Natacha Heutte, Anne El Kaddoumi, et al.. Recur- rence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed. Annals of Agricultural and Environmental Medicine (AAEM), Institute of Agricultural Medicine in Lublin, 2012, 19 (1), pp.61-67. hal-02186772 HAL Id: hal-02186772 https://hal-normandie-univ.archives-ouvertes.fr/hal-02186772 Submitted on 13 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Annals of Agricultural and Environmental Medicine 2012, Vol 19, No 1, 61-67 ORIGINAL ARTICLE www.aaem.pl Recurrence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed Caroline Lanier1, Véronique André1, Virginie Séguin1, Natacha Heutte1, Anne El Kaddoumi1, Valérie Bouchart2, Rachel Picquet2, David Garon1 1 Université de Caen Basse Normandie, Caen, France 2 Laboratoire Départemental Frank Duncombe, Conseil Général du Calvados, Saint Contest, France Lanier C, André V, Séguin V, Heutte N, Kaddoumi A, Bouchart V, Picquet R, Garon D. -

HHE Report No. HETA-1999-0014-3094, Springdale
This Health Hazard Evaluation (HHE) report and any recommendations made herein are for the specific facility evaluated and may not be universally applicable. Any recommendations made are not to be considered as final statements of NIOSH policy or of any agency or individual involved. Additional HHE reports are available at http://www.cdc.gov/niosh/hhe/ Workplace Safety and Health Evaluation of Mold Contamination in a Hotel Before and After Remediation Kenneth Martinez, CIH, MSEE Douglas Trout, MD, MHS Kenneth Wallingford, MS, CIH Chandran Achutan, PhD Mitchell Singal, MD, MPH Health Hazard Evaluation Report HETA 1999-0014-3094 Springdale Best Western Hotel Cincinnati, Ohio November 2009 Department of Health and Human Services Centers for Disease Control and Prevention National Institute for Occupational Safety and Health The employer shall post a copy of this report for a period of 30 calendar days at or near the workplace(s) of affected employees. The employer shall take steps to insure that the posted determinations are not altered, defaced, or covered by other material during such period. [37 FR 23640, November 7, 1972, as amended at 45 FR 2653, January 14, 1980]. CONTENTS REPO R T Highlights of the NIOSH Health Hazard Evaluation .............ii Summary ............................................................................ iii Introduction ..........................................................................1 Background .........................................................................1 Methods ...............................................................................2 -

Orthologous Allergens and Diagnostic Utility of Major Allergen Alt
Original Article Allergy Asthma Immunol Res. 2016 September;8(5):428-437. http://dx.doi.org/10.4168/aair.2016.8.5.428 pISSN 2092-7355 • eISSN 2092-7363 Orthologous Allergens and Diagnostic Utility of Major Allergen Alt a 1 Antonio Moreno,1 Fernando Pineda,2* Javier Alcover,2 David Rodríguez,2 Ricardo Palacios,2 Eduardo Martínez-Naves3 1Allergy Service, Hospital of Cuenca, Cuenca, Spain 2DIATER laboratories, Madrid, Spain 3Department of Microbiology, School of Medicine, University Complutense of Madrid, Madrid, Spain This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose: Hypersensitivity to fungi is associated with rhinoconjunctivitis and asthma. For some fungi, such as Alternaria alternata (A. alternata), the symptoms of asthma are persistent, increasing disease severity and the risk of fatal outcomes. There are a large number of species of fungi but knowledge of them remains limited. This, together with the difficulties in obtaining adequate standardized extracts, means that there remain signifi- cant challenges in the diagnosis and immunotherapy of allergy associated with fungi. The type of indoor fungi related to asthma/allergy varies ac- cording to geographic, climatic, and seasonal factors, making their study difficult. The aim of this study was to determine hypersensitivity to indoor fungi in a population from Cuenca, Spain. Methods: Thirty-five patients with symptoms compatible with rhinitis or asthma who showed clear wors- ening of their symptoms in their homes or workplace were included. -

The Effect of Spaceflight on Growth of Ulocladium Chartarum Colonies on the International Space Station
The Effect of Spaceflight on Growth of Ulocladium chartarum Colonies on the International Space Station Ioana Gomoiu1*, Elias Chatzitheodoridis2, Sonia Vadrucci3, Isabelle Walther3 1 Institute of Biology Bucharest, Romanian Academy of Science, Bucharest, Romania, 2 School of Mining and Metallurgical Engineering, National Technical University of Athens, Athens, Greece, 3 Space Biology Group, ETH Zu¨rich, Zu¨rich, Switzerland Abstract The objectives of this 14 days experiment were to investigate the effect of spaceflight on the growth of Ulocladium chartarum, to study the viability of the aerial and submerged mycelium and to put in evidence changes at the cellular level. U. chartarum was chosen for the spaceflight experiment because it is well known to be involved in biodeterioration of organic and inorganic substrates covered with organic deposits and expected to be a possible contaminant in Spaceships. Colonies grown on the International Space Station (ISS) and on Earth were analysed post-flight. This study clearly indicates that U. chartarum is able to grow under spaceflight conditions developing, as a response, a complex colony morphotype never mentioned previously. We observed that spaceflight reduced the rate of growth of aerial mycelium, but stimulated the growth of submerged mycelium and of new microcolonies. In Spaceships and Space Stations U. chartarum and other fungal species could find a favourable environment to grow invasively unnoticed in the depth of surfaces containing very small amount of substrate, posing a risk factor for biodegradation of structural components, as well as a direct threat for crew health. The colony growth cycle of U. chartarum provides a useful eukaryotic system for the study of fungal growth under spaceflight conditions. -

Index of Fungal Names
INDEX OF FUNGAL NAMES Alternaria cerealis 187 Alternaria cetera 188–189 Alphabetical list of fungal species, genera and families treated in Alternaria chartarum 201 the Taxonomy sections of the included manuscripts. Alternaria chartarum f. stemphylioides 201 Alternaria cheiranthi 189 A Alternaria chlamydospora 190, 199 Alternaria chlamydosporigena 190 Acicuseptoria 376–377 Alternaria “chlamydosporum” 199 Acicuseptoria rumicis 376–377 Alternaria chrysanthemi 204 Allantozythia 384 Alternaria cichorii 200 Allewia 183 Alternaria cinerariae 202 Allewia eureka 193 Alternaria cinerea 207 Allewia proteae 193 Alternaria cirsinoxia 200 Alternaria 183, 186, 190, 193, 198, 207 Alternaria citriarbusti 187 Alternaria abundans 189 Alternaria citrimacularis 187 Alternaria acalyphicola 200 Alternaria colombiana 187 Alternaria agerati 200 Alternaria concatenata 201 Alternaria agripestis 200 Alternaria conjuncta 196 Alternaria allii 191 Alternaria conoidea 188 Alternaria alternantherae 185 Alternaria “consortiale” 204 Alternaria alternariae 206 Alternaria consortialis 204 Alternaria alternarina 195 Alternaria crassa 200 Alternaria cretica 200 Alternaria alternata 183, 185–186 Alternaria cucumerina 200 Alternaria anagallidis 200 Alternaria cucurbitae 204 Alternaria angustiovoidea 187 Alternaria cumini 193 Alternaria anigozanthi 193 Alternaria cyphomandrae 201 Alternaria aragakii 200 Alternaria danida 201 Alternaria araliae 199 Alternaria dauci 201 Alternaria arborescens 187, 201 Alternaria daucicaulis 196 Alternaria arbusti 195 Alternaria daucifollii 187 -

Diversity and Control of Spoilage Fungi in Dairy Products: an Update
microorganisms Review Diversity and Control of Spoilage Fungi in Dairy Products: An Update Lucille Garnier 1,2 ID , Florence Valence 2 and Jérôme Mounier 1,* 1 Laboratoire Universitaire de Biodiversité et Ecologie Microbienne (LUBEM EA3882), Université de Brest, Technopole Brest-Iroise, 29280 Plouzané, France; [email protected] 2 Science et Technologie du Lait et de l’Œuf (STLO), AgroCampus Ouest, INRA, 35000 Rennes, France; fl[email protected] * Correspondence: [email protected]; Tel.: +33-(0)2-90-91-51-00; Fax: +33-(0)2-90-91-51-01 Received: 10 July 2017; Accepted: 25 July 2017; Published: 28 July 2017 Abstract: Fungi are common contaminants of dairy products, which provide a favorable niche for their growth. They are responsible for visible or non-visible defects, such as off-odor and -flavor, and lead to significant food waste and losses as well as important economic losses. Control of fungal spoilage is a major concern for industrials and scientists that are looking for efficient solutions to prevent and/or limit fungal spoilage in dairy products. Several traditional methods also called traditional hurdle technologies are implemented and combined to prevent and control such contaminations. Prevention methods include good manufacturing and hygiene practices, air filtration, and decontamination systems, while control methods include inactivation treatments, temperature control, and modified atmosphere packaging. However, despite technology advances in existing preservation methods, fungal spoilage is still an issue for dairy manufacturers and in recent years, new (bio) preservation technologies are being developed such as the use of bioprotective cultures. This review summarizes our current knowledge on the diversity of spoilage fungi in dairy products and the traditional and (potentially) new hurdle technologies to control their occurrence in dairy foods. -

Health Forest Tree Seed Health for Germplasm Conservation 2010-TPFQ-16 Agenda: 14.1 Forest Tree Seed Health for Germplasm Conservation
IPGRI TECHNICAL BULLETIN NO. 6 Forest tree seed health tree Forest Forest Tree Seed Health For Germplasm Conservation 2010-TPFQ-16 Agenda: 14.1 Forest tree seed health for germplasm conservation J.R. Sutherland, M. Diekmann and P. Berjak, editors Technical Bulletin No. 6 Technical IPGRI is a Future Harvest Centre supported by the Consultative Group on International Agricultural Research (CGIAR) IPGRI ISBN 92-9043-515-1 Page 1 of 89 Forest Tree Seed Health For Germplasm Conservation 2010-TPFQ-16 Agenda: 14.1 IPGRI Technical Bulletins are published by the International Plant Genetic Resources Institute with the intention of putting forward definitive recommendations for techniques in genetic resources. They are specifically aimed at National Programme and genebank personnel. Previous titles in this series: A protocol to determine seed storage behaviour T.D. Hong and R.H. Ellis IPGRI Technical Bulletin No. 1, 1996. Molecular tools in plant genetic resources conservation: a guide to the technologies A. Karp, S. Kresovich, K.V. Bhat, W.G. Ayad and T. Hodgkin IPGRI Technical Bulletin No. 2, 1997. Core collections of plant genetic resources Th.J.L. van Hintum, A.H.D. Brown, C. Spillane and T. Hodgkin IPGRI Technical Bulletin No. 3, 2000. Design and analysis of evaluation trials of genetic resources collections Statistical Services Centre and University of Reading IPGRI Technical Bulletin No. 4, 2001. Accession management: combining or splitting accessions as a tool to improve germplasm management efficiency N.R. Sackville Hamilton, J.M.M. Engels, Th.J.L. van Hintum, B. Koo and M. Smale IPGRI Technical Bulletin No. -

Determination of the Reference Genes for Qrt-PCR Normalization and Expression Levels of MAT Genes Under Various Conditions in Ulocladium
Determination of the reference genes for qRT-PCR normalization and expression levels of MAT genes under various conditions in Ulocladium Li-Guo Ma1 and Yun Geng2 1 Shandong Key Laboratory of Plant Virology, Institute of Plant Protection, Shandong Academy of Agricultural Sciences, Jinan, China 2 Biotechnology Research Center, Shandong Academy of Agricultural Sciences, Jinan, China ABSTRACT The genus Ulocladium is thought to be strictly asexual. One of the possible reasons for the lack of sexuality in Ulocladium species is the absence of the stimulus of environmental factors. Sexual reproduction in ascomycetes is controlled by a specific region in the genome referred to as mating-type locus (MAT) that consists of two dissimilar DNA sequences in the mating partners, termed MAT1-1 and MAT1-2 idiomorphs. To identify the response of MAT loci to environmental conditions, the mRNA transcription level of MAT1-1-1 and MAT1-2-1 genes was tested using qRT-PCR under different temperatures (−20 C, −10 C, 0 C, 10 C, 20 C, 30 C and 40 C), culture medias (CM, OA, HAY, PCA, PDA and V8), photoperiods (24 h light, 24 h dark, 12 h light/12 h dark, 10 h light/14 h dark and 8 h light/16 h dark), and CO2 concentrations (0.03%, 0.5%, 1%, 5%, 10%, 15% and 20%). For obtaining reliable results from qRT-PCR, the most stable internal control gene and optimal number of reference genes for normalization were determined under different treatments. The results showed that there is no universal internal control gene that is expressed at a constant level under different experimental treatments.