Recurrence of Stachybotrys Chartarum During Mycological And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

43-09-405 and 407 Final Combine
Pak. J. Bot ., 44(6): 2093-2102, 2012. A NEW SPECIES OF TIAROSPORELLA AZADARICHTA AND NEW FUNGAL RECORDS ON AZADIRACHTA INDICA FROM PAKISTAN SYED QAISER ABBAS 1, TEHREEMA IFTIKHAR 1* , MUBASHIR NIAZ 1, IFTIKHAR ALI 1, NABILA IFTIKHAR 1 AND ALIA ABBAS 2 1Department of Botany, Government College University, Alama Iqbal Road, Faisalabad, Pakistan 2Department of Botany, Federal Urdu University, Karachi, Pakistan *Corresponding author’s e-mail: [email protected] Abstract A new species of Tiarosporella azadarichta has been described on Azadirachta indica and some new fungal records viz ., Diplozythiella bambusina , Ulocladium chartarum , Cladosporium nigrellum, Cladosporium oxysporum. Didymostilbe coffeae , Muellerella pygmaea, Lasiodiplodia paraphysaria, Monochaetinula terminalae, Trimmatostroma sp., and Epidermophyton floccosum are reported on Azadirachta indica for the first time from Pakistan. Introduction reason a detailed survey of the plant for fungi was under taken in this study. Azadirachta indica (as Azadirachta Juss, Melia azadirachta ) belong to the family Meliaceae. Neem is its Materials and Methods common name. It is important due to its commercial and medicinal value. Azadirachta indica is also an important Samples of Azadirachta indica were collected from plant for its potential of anti-fungal, anti-bacterial and the different areas of District Faisalabad and Gojra. The insecticidal activity. Decline of trees due to fungi are different areas were G.C. University. Faisalabad, increasing tremendously in Pakistan especially in Punjab Agriculture University Faisalabad, Gutwala forest (park) and Sindh (Javed et al ., 2004; Khanzada et al ., 2011; Fateh Faisalabad, Tandlianwala and Gojra City. Methods and et al., 2011; Rheman et al., 2011; Farooq et al., 2011). materials are the same as described Abbas et al ., (2010). -

An Unusual Haemoid Fungi: Ulocladium, As a Cause Of
Volume 2 Number 2 (June 2010) 95-97 CASE Report An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis Kaur R, Wadhwa A1,Gulati A2, Agrawal AK2 1Department of Microbiology. 2Department of Otorhinolaryngology, Maulana Azad Medical College, New Delhi, India. Received: April 2010, Accepted: May 2010. ABSTRACT Allergic fungal sinusitis (AFS) has been recognized as an important cause of chronic sinusitis commonly caused by Aspergillus spp. and various dematiaceous fungi like Bipolaris, Alternaria, Curvalaria, and etc. Ulocladium botrytis is a non pathogenic environmental dematiaceous fungi, which has been recently described as a human pathogen. Ulocladium has never been associated with allergic fungal sinusitis but it was identified as an etiological agent of AFS in a 35 year old immunocompetent female patient presenting with chronic nasal obstruction of several months duration to our hospital. The patient underwent FESS and the excised polyps revealed Ulocladium as the causative fungal agent. Keywords: Ulocladium, Phaeoid Fungi, Chronic Sinusitis. INTRODUCTION CASE REPORT Chronic allergic sinusitis is a common condition A 35 year old female patient presented to the ENT responsible for the development of nasal polyps, out patient department of the Lok Nayak Hospital, described as abnormal lesions that emanate from Delhi, with chronic nasal obstruction, excessive any portion of the nasal mucosa or Para nasal sneezing, nasal discharge and frontal headache since sinuses. They are commonly located in the middle several months. Nasal obstruction was of gradual meatus and ethmoid sinus and are present in onset, non progressive, more on the left side than 1-4% of the population (1). -
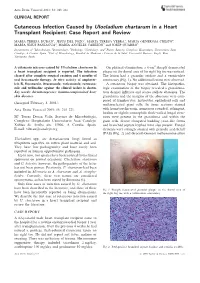
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Mould and Yeast Identification in Archival Settings
International Biodeterioration & Biodegradation 65 (2011) 619e627 Contents lists available at ScienceDirect International Biodeterioration & Biodegradation journal homepage: www.elsevier.com/locate/ibiod Mould and yeast identification in archival settings: Preliminary results on the use of traditional methods and molecular biology options in Portuguese archives A.C. Pinheiro a,*, M.F. Macedo b, V. Jurado c, C. Saiz-Jimenez c, C. Viegas d, J. Brandão e, L. Rosado e a Departamento de Conservação e Restauro da Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, Portugal b Vicarte, Departamento de Conservação e Restauro da Faculdade Ciências e Tecnologia da Universidade Nova de Lisboa, Portugal c Instituto de Recursos Naturales y Agrobiologia, CSIC, Sevilla, Spain d Escola Superior de Tecnologias de Saúde de Lisboa, Instituto Politécnico de Lisboa, Portugal e Unidade de Referência de Doenças Sistémicas e Zoonoses do Departamento de Doenças Infecciosas do Instituto Nacional de Saúde Doutor Ricardo Jorge, IP, Lisboa, Portugal article info abstract Article history: This project was developed to fully assess the indoor air quality in archives and libraries from a fungal flora Received 14 September 2010 point of view. It uses classical methodologies such as traditional culture media e for the viable fungi e and Received in revised form modern molecular biology protocols, especially relevant to assess the non-viable fraction of the biological 4 February 2011 contaminants. Denaturing high-performance liquid chromatography (DHPLC) has emerged as an alter- Accepted 4 February 2011 native to denaturing gradient gel electrophoresis (DGGE) and has already been applied to the study of Available online 12 April 2011 a few bacterial communities. -

Nomination Background: Stachybotrys Chartarum Strain 2 Mold (Atranone Chemotype) (CASRN: STACHYSTRN2)
Stachybotrys chartarum (or S. atra or S. alternans) [CAS No. 67892-26-6] Review of Toxicological Literature June 2004 Stachybotrys chartarum (or S. atra or S. alternans) [CAS No. 67892-26-6] Review of Toxicological Literature Prepared for National Toxicology Program (NTP) National Institute of Environmental Health Sciences (NIEHS) National Institutes of Health U.S Department of Health and Human Services Contract No. N01-ES-35515 Project Officer: Scott A. Masten, Ph.D. NTP/NIEHS Research Triangle Park, North Carolina Prepared by Integrated Laboratory Systems, Inc. Research Triangle Park, North Carolina June 2004 Abstract Stachybotrys chartarum is a greenish-black mold in the fungal division Deuteromycota, a catch-all group for fungi for which a sexually reproducing stage is unknown. It produces asexual spores (conidia). The morphology and color of conidia and other structures examined microscopically help distinguish the species from other molds found in indoor air that may contaminate materials in buildings that have suffered water intrusion. S. chartarum may ultimately overgrow other molds that have also produced colonies on wet cellulosic materials such as drywall (gypsum board, wallboard, sheet rock, etc.). Because of the likelihood that it may produce toxic macrocyclic trichothecenes and hemolytic stachylysin (exposure to which may be associated with idiopathic pulmonary hemorrhage [IPH] in infants), S. chartarum exposure is of concern to the members of the general public whose homes and workplaces have been contaminated after water intrusion, to agricultural and textile workers who handle contaminated plant material, and to workers involved in remediation of mold-damaged structures. Dry conidia, hyphae, and other fragments can be mechanically aerosolized as inhalable particulates. -

Monograph on Dematiaceous Fungi
Monograph On Dematiaceous fungi A guide for description of dematiaceous fungi fungi of medical importance, diseases caused by them, diagnosis and treatment By Mohamed Refai and Heidy Abo El-Yazid Department of Microbiology, Faculty of Veterinary Medicine, Cairo University 2014 1 Preface The first time I saw cultures of dematiaceous fungi was in the laboratory of Prof. Seeliger in Bonn, 1962, when I attended a practical course on moulds for one week. Then I handled myself several cultures of black fungi, as contaminants in Mycology Laboratory of Prof. Rieth, 1963-1964, in Hamburg. When I visited Prof. DE Varies in Baarn, 1963. I was fascinated by the tremendous number of moulds in the Centraalbureau voor Schimmelcultures, Baarn, Netherlands. On the other hand, I was proud, that El-Sheikh Mahgoub, a Colleague from Sundan, wrote an internationally well-known book on mycetoma. I have never seen cases of dematiaceous fungal infections in Egypt, therefore, I was very happy, when I saw the collection of mycetoma cases reported in Egypt by the eminent Egyptian Mycologist, Prof. Dr Mohamed Taha, Zagazig University. To all these prominent mycologists I dedicate this monograph. Prof. Dr. Mohamed Refai, 1.5.2014 Heinz Seeliger Heinz Rieth Gerard de Vries, El-Sheikh Mahgoub Mohamed Taha 2 Contents 1. Introduction 4 2. 30. The genus Rhinocladiella 83 2. Description of dematiaceous 6 2. 31. The genus Scedosporium 86 fungi 2. 1. The genus Alternaria 6 2. 32. The genus Scytalidium 89 2.2. The genus Aurobasidium 11 2.33. The genus Stachybotrys 91 2.3. The genus Bipolaris 16 2. -

HHE Report No. HETA-1999-0014-3094, Springdale
This Health Hazard Evaluation (HHE) report and any recommendations made herein are for the specific facility evaluated and may not be universally applicable. Any recommendations made are not to be considered as final statements of NIOSH policy or of any agency or individual involved. Additional HHE reports are available at http://www.cdc.gov/niosh/hhe/ Workplace Safety and Health Evaluation of Mold Contamination in a Hotel Before and After Remediation Kenneth Martinez, CIH, MSEE Douglas Trout, MD, MHS Kenneth Wallingford, MS, CIH Chandran Achutan, PhD Mitchell Singal, MD, MPH Health Hazard Evaluation Report HETA 1999-0014-3094 Springdale Best Western Hotel Cincinnati, Ohio November 2009 Department of Health and Human Services Centers for Disease Control and Prevention National Institute for Occupational Safety and Health The employer shall post a copy of this report for a period of 30 calendar days at or near the workplace(s) of affected employees. The employer shall take steps to insure that the posted determinations are not altered, defaced, or covered by other material during such period. [37 FR 23640, November 7, 1972, as amended at 45 FR 2653, January 14, 1980]. CONTENTS REPO R T Highlights of the NIOSH Health Hazard Evaluation .............ii Summary ............................................................................ iii Introduction ..........................................................................1 Background .........................................................................1 Methods ...............................................................................2 -

Orthologous Allergens and Diagnostic Utility of Major Allergen Alt
Original Article Allergy Asthma Immunol Res. 2016 September;8(5):428-437. http://dx.doi.org/10.4168/aair.2016.8.5.428 pISSN 2092-7355 • eISSN 2092-7363 Orthologous Allergens and Diagnostic Utility of Major Allergen Alt a 1 Antonio Moreno,1 Fernando Pineda,2* Javier Alcover,2 David Rodríguez,2 Ricardo Palacios,2 Eduardo Martínez-Naves3 1Allergy Service, Hospital of Cuenca, Cuenca, Spain 2DIATER laboratories, Madrid, Spain 3Department of Microbiology, School of Medicine, University Complutense of Madrid, Madrid, Spain This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose: Hypersensitivity to fungi is associated with rhinoconjunctivitis and asthma. For some fungi, such as Alternaria alternata (A. alternata), the symptoms of asthma are persistent, increasing disease severity and the risk of fatal outcomes. There are a large number of species of fungi but knowledge of them remains limited. This, together with the difficulties in obtaining adequate standardized extracts, means that there remain signifi- cant challenges in the diagnosis and immunotherapy of allergy associated with fungi. The type of indoor fungi related to asthma/allergy varies ac- cording to geographic, climatic, and seasonal factors, making their study difficult. The aim of this study was to determine hypersensitivity to indoor fungi in a population from Cuenca, Spain. Methods: Thirty-five patients with symptoms compatible with rhinitis or asthma who showed clear wors- ening of their symptoms in their homes or workplace were included. -

Savoryellales (Hypocreomycetidae, Sordariomycetes): a Novel Lineage
Mycologia, 103(6), 2011, pp. 1351–1371. DOI: 10.3852/11-102 # 2011 by The Mycological Society of America, Lawrence, KS 66044-8897 Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella Nattawut Boonyuen1 Canalisporium) formed a new lineage that has Mycology Laboratory (BMYC), Bioresources Technology invaded both marine and freshwater habitats, indi- Unit (BTU), National Center for Genetic Engineering cating that these genera share a common ancestor and Biotechnology (BIOTEC), 113 Thailand Science and are closely related. Because they show no clear Park, Phaholyothin Road, Khlong 1, Khlong Luang, Pathumthani 12120, Thailand, and Department of relationship with any named order we erect a new Plant Pathology, Faculty of Agriculture, Kasetsart order Savoryellales in the subclass Hypocreomyceti- University, 50 Phaholyothin Road, Chatuchak, dae, Sordariomycetes. The genera Savoryella and Bangkok 10900, Thailand Ascothailandia are monophyletic, while the position Charuwan Chuaseeharonnachai of Ascotaiwania is unresolved. All three genera are Satinee Suetrong phylogenetically related and form a distinct clade Veera Sri-indrasutdhi similar to the unclassified group of marine ascomy- Somsak Sivichai cetes comprising the genera Swampomyces, Torpedos- E.B. Gareth Jones pora and Juncigera (TBM clade: Torpedospora/Bertia/ Mycology Laboratory (BMYC), Bioresources Technology Melanospora) in the Hypocreomycetidae incertae -

Stachybotrys Musae Sp. Nov., S. Microsporus, and Memnoniella Levispora (Stachybotryaceae, Hypocreales) Found on Bananas in China and Thailand
life Article Stachybotrys musae sp. nov., S. microsporus, and Memnoniella levispora (Stachybotryaceae, Hypocreales) Found on Bananas in China and Thailand Binu C. Samarakoon 1,2,3, Dhanushka N. Wanasinghe 1,4,5, Rungtiwa Phookamsak 1,4,5,6 , Jayarama Bhat 7, Putarak Chomnunti 2,3, Samantha C. Karunarathna 1,4,5,6,* and Saisamorn Lumyong 6,8,9,* 1 CAS Key Laboratory for Plant Biodiversity and Biogeography of East Asia (KLPB), Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; [email protected] (B.C.S.); [email protected] (D.N.W.); [email protected] (R.P.) 2 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand; [email protected] 3 School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 4 World Agroforestry Centre, East and Central Asia, 132 Lanhei Road, Kunming 650201, China 5 Centre for Mountain Futures (CMF), Kunming Institute of Botany, Kunming 650201, China 6 Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Sciences, Chiang Mai University, Chiang Mai 50200, Thailand 7 Formerly, Department of Botany, Goa University, Goa, Res: House No. 128/1-J, Azad Co-Op Housing Society, Curca, P.O. Goa Velha 403108, India; [email protected] 8 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand 9 Academy of Science, The Royal Society of Thailand, Bangkok 10300, Thailand * Correspondence: [email protected] (S.C.K.); [email protected] (S.L.) Citation: Samarakoon, B.C.; Wanasinghe, D.N.; Phookamsak, R.; Abstract: A study was conducted to investigate saprobic fungal niches of Stachybotryaceae (Hypocre- Bhat, J.; Chomnunti, P.; Karunarathna, ales) associated with leaves of Musa (banana) in China and Thailand. -

Molds: How Can You Protect Your Operation from Them?
MOLDS: HOW CAN YOU PROTECT YOUR OPERATION FROM THEM? J.J. Morrell Oregon State University Corvallis, Oregon Introduction In recent months, newspapers and talk shows have been filled with reports of toxic molds in houses that sicken occupants, kill pets, and like anything else in the U.S., lead to litigation. Wood products have been implicated in a number of these instances, but there is little evidence that wood is the main culprit. So what can you do to reduce your risks? How should you respond to customer concerns? What can you do to ensure that the material you produce is mold free? In order to answer all these questions, this report will provide some background about fungi and their growth on wood, then discuss methods for preventing growth of these fungi. Fungi on Wood Wood products can be colonized by a variety of fungi. Molds are primary colonizers that grow on freshly exposed surfaces of a variety of materials including paints, food, and wood. The damage caused by these fungi is mainly cosmetic due to the presence of pigmented spores. Stain fungi are also primary colonizers, but their discoloration extends more deeply into the wood. Decay fungi are usually secondary colonizers (meaning that they enter the wood some time after it is harvested), and they damage the structural polymers that make up the wood While wood has been used for thousands of years, there is relatively little information on the types of fungi colonizing a given wood species. One recent study found that Douglas-fir sapwood was colonized by over 45 species and that colonization increased with storage time. -

The Effect of Spaceflight on Growth of Ulocladium Chartarum Colonies on the International Space Station
The Effect of Spaceflight on Growth of Ulocladium chartarum Colonies on the International Space Station Ioana Gomoiu1*, Elias Chatzitheodoridis2, Sonia Vadrucci3, Isabelle Walther3 1 Institute of Biology Bucharest, Romanian Academy of Science, Bucharest, Romania, 2 School of Mining and Metallurgical Engineering, National Technical University of Athens, Athens, Greece, 3 Space Biology Group, ETH Zu¨rich, Zu¨rich, Switzerland Abstract The objectives of this 14 days experiment were to investigate the effect of spaceflight on the growth of Ulocladium chartarum, to study the viability of the aerial and submerged mycelium and to put in evidence changes at the cellular level. U. chartarum was chosen for the spaceflight experiment because it is well known to be involved in biodeterioration of organic and inorganic substrates covered with organic deposits and expected to be a possible contaminant in Spaceships. Colonies grown on the International Space Station (ISS) and on Earth were analysed post-flight. This study clearly indicates that U. chartarum is able to grow under spaceflight conditions developing, as a response, a complex colony morphotype never mentioned previously. We observed that spaceflight reduced the rate of growth of aerial mycelium, but stimulated the growth of submerged mycelium and of new microcolonies. In Spaceships and Space Stations U. chartarum and other fungal species could find a favourable environment to grow invasively unnoticed in the depth of surfaces containing very small amount of substrate, posing a risk factor for biodegradation of structural components, as well as a direct threat for crew health. The colony growth cycle of U. chartarum provides a useful eukaryotic system for the study of fungal growth under spaceflight conditions.