The Effect of Spaceflight on Growth of Ulocladium Chartarum Colonies on the International Space Station
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

43-09-405 and 407 Final Combine
Pak. J. Bot ., 44(6): 2093-2102, 2012. A NEW SPECIES OF TIAROSPORELLA AZADARICHTA AND NEW FUNGAL RECORDS ON AZADIRACHTA INDICA FROM PAKISTAN SYED QAISER ABBAS 1, TEHREEMA IFTIKHAR 1* , MUBASHIR NIAZ 1, IFTIKHAR ALI 1, NABILA IFTIKHAR 1 AND ALIA ABBAS 2 1Department of Botany, Government College University, Alama Iqbal Road, Faisalabad, Pakistan 2Department of Botany, Federal Urdu University, Karachi, Pakistan *Corresponding author’s e-mail: [email protected] Abstract A new species of Tiarosporella azadarichta has been described on Azadirachta indica and some new fungal records viz ., Diplozythiella bambusina , Ulocladium chartarum , Cladosporium nigrellum, Cladosporium oxysporum. Didymostilbe coffeae , Muellerella pygmaea, Lasiodiplodia paraphysaria, Monochaetinula terminalae, Trimmatostroma sp., and Epidermophyton floccosum are reported on Azadirachta indica for the first time from Pakistan. Introduction reason a detailed survey of the plant for fungi was under taken in this study. Azadirachta indica (as Azadirachta Juss, Melia azadirachta ) belong to the family Meliaceae. Neem is its Materials and Methods common name. It is important due to its commercial and medicinal value. Azadirachta indica is also an important Samples of Azadirachta indica were collected from plant for its potential of anti-fungal, anti-bacterial and the different areas of District Faisalabad and Gojra. The insecticidal activity. Decline of trees due to fungi are different areas were G.C. University. Faisalabad, increasing tremendously in Pakistan especially in Punjab Agriculture University Faisalabad, Gutwala forest (park) and Sindh (Javed et al ., 2004; Khanzada et al ., 2011; Fateh Faisalabad, Tandlianwala and Gojra City. Methods and et al., 2011; Rheman et al., 2011; Farooq et al., 2011). materials are the same as described Abbas et al ., (2010). -

An Unusual Haemoid Fungi: Ulocladium, As a Cause Of
Volume 2 Number 2 (June 2010) 95-97 CASE Report An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis Kaur R, Wadhwa A1,Gulati A2, Agrawal AK2 1Department of Microbiology. 2Department of Otorhinolaryngology, Maulana Azad Medical College, New Delhi, India. Received: April 2010, Accepted: May 2010. ABSTRACT Allergic fungal sinusitis (AFS) has been recognized as an important cause of chronic sinusitis commonly caused by Aspergillus spp. and various dematiaceous fungi like Bipolaris, Alternaria, Curvalaria, and etc. Ulocladium botrytis is a non pathogenic environmental dematiaceous fungi, which has been recently described as a human pathogen. Ulocladium has never been associated with allergic fungal sinusitis but it was identified as an etiological agent of AFS in a 35 year old immunocompetent female patient presenting with chronic nasal obstruction of several months duration to our hospital. The patient underwent FESS and the excised polyps revealed Ulocladium as the causative fungal agent. Keywords: Ulocladium, Phaeoid Fungi, Chronic Sinusitis. INTRODUCTION CASE REPORT Chronic allergic sinusitis is a common condition A 35 year old female patient presented to the ENT responsible for the development of nasal polyps, out patient department of the Lok Nayak Hospital, described as abnormal lesions that emanate from Delhi, with chronic nasal obstruction, excessive any portion of the nasal mucosa or Para nasal sneezing, nasal discharge and frontal headache since sinuses. They are commonly located in the middle several months. Nasal obstruction was of gradual meatus and ethmoid sinus and are present in onset, non progressive, more on the left side than 1-4% of the population (1). -
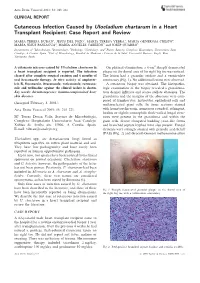
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Novel Dematiaceous Hyphomycetes
STUDIES IN MYCOLOGY 50: 109–118. 2004. Novel dematiaceous hyphomycetes * Emory G. Simmons 717 Thornwood Road, Crawfordsville, Indiana 47933, U.S.A. *Correspondence: Emory G. Simmons, [email protected] Abstract: Novel taxa are described as Alternaria simsimi isolated from Sesamum indicum, distinguishing it from Alternaria sesami; Embellisia lolii from Lolium perenne; Nimbya perpunctulata from Alternanthera philoxeroides; Ulocladium arbores- cens from Pistacia vera; and Stemphylium symphyti from Symphytum × uplandicum. Taxonomic novelties: Alternaria simsimi E.G. Simmons sp. nov., Embellisia lolii E.G. Simmons & C.F. Hill sp. nov., Nimbya perpunctulata E.G. Simmons sp. nov., Stemphylium symphyti E.G. Simmons sp. nov., Ulocladium arborescens E.G. Simmons sp. nov. Key words: Alternaria, Embellisia, Nimbya, Ulocladium, Stemphylium, anamorphs, Sesamum, Lolium, Alternanthera, Pistacia, Symphytum. INTRODUCTION described and discussed on the basis of examination of colonies at 50× magnification and, at higher magnifi- It is a pleasure to be able to offer several new taxa in cations, of sporulation elements sampled at 5–7 d after honour of the centenary observances of the Centraal- inoculation. Each isolate or specimen discussed is bureau voor Schimmelcultures. These are dedicated to identified by a unique record/research number in the mycologist colleagues who were among my first format E.G.S. 00.000. associates during my earliest visits to CBS in Baarn, beginning in the late 1950s: Agathe van Beverwijk, Gerharda Bunschoten, G.A. de Vries, Amelia Stolk, TAXONOMIC PART Beatrice Schol-Schwarz, Maria Schipper, and Annie van der Plaats-Niterink. A novel taxon is presented for Alternaria: three species on sesame, two as patho- each of the five hyphomycetous genera that have been gens the major focus of my taxonomic work over the past Alternaria isolates from invaded tissues of Sesamum 50+ years. -

Monograph on Dematiaceous Fungi
Monograph On Dematiaceous fungi A guide for description of dematiaceous fungi fungi of medical importance, diseases caused by them, diagnosis and treatment By Mohamed Refai and Heidy Abo El-Yazid Department of Microbiology, Faculty of Veterinary Medicine, Cairo University 2014 1 Preface The first time I saw cultures of dematiaceous fungi was in the laboratory of Prof. Seeliger in Bonn, 1962, when I attended a practical course on moulds for one week. Then I handled myself several cultures of black fungi, as contaminants in Mycology Laboratory of Prof. Rieth, 1963-1964, in Hamburg. When I visited Prof. DE Varies in Baarn, 1963. I was fascinated by the tremendous number of moulds in the Centraalbureau voor Schimmelcultures, Baarn, Netherlands. On the other hand, I was proud, that El-Sheikh Mahgoub, a Colleague from Sundan, wrote an internationally well-known book on mycetoma. I have never seen cases of dematiaceous fungal infections in Egypt, therefore, I was very happy, when I saw the collection of mycetoma cases reported in Egypt by the eminent Egyptian Mycologist, Prof. Dr Mohamed Taha, Zagazig University. To all these prominent mycologists I dedicate this monograph. Prof. Dr. Mohamed Refai, 1.5.2014 Heinz Seeliger Heinz Rieth Gerard de Vries, El-Sheikh Mahgoub Mohamed Taha 2 Contents 1. Introduction 4 2. 30. The genus Rhinocladiella 83 2. Description of dematiaceous 6 2. 31. The genus Scedosporium 86 fungi 2. 1. The genus Alternaria 6 2. 32. The genus Scytalidium 89 2.2. The genus Aurobasidium 11 2.33. The genus Stachybotrys 91 2.3. The genus Bipolaris 16 2. -

PERSOONIAL R Eflections
Persoonia 23, 2009: 177–208 www.persoonia.org doi:10.3767/003158509X482951 PERSOONIAL R eflections Editorial: Celebrating 50 years of Fungal Biodiversity Research The year 2009 represents the 50th anniversary of Persoonia as the message that without fungi as basal link in the food chain, an international journal of mycology. Since 2008, Persoonia is there will be no biodiversity at all. a full-colour, Open Access journal, and from 2009 onwards, will May the Fungi be with you! also appear in PubMed, which we believe will give our authors even more exposure than that presently achieved via the two Editors-in-Chief: independent online websites, www.IngentaConnect.com, and Prof. dr PW Crous www.persoonia.org. The enclosed free poster depicts the 50 CBS Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT most beautiful fungi published throughout the year. We hope Utrecht, The Netherlands. that the poster acts as further encouragement for students and mycologists to describe and help protect our planet’s fungal Dr ME Noordeloos biodiversity. As 2010 is the international year of biodiversity, we National Herbarium of the Netherlands, Leiden University urge you to prominently display this poster, and help distribute branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands. Book Reviews Mu«enko W, Majewski T, Ruszkiewicz- The Cryphonectriaceae include some Michalska M (eds). 2008. A preliminary of the most important tree pathogens checklist of micromycetes in Poland. in the world. Over the years I have Biodiversity of Poland, Vol. 9. Pp. personally helped collect populations 752; soft cover. Price 74 €. W. Szafer of some species in Africa and South Institute of Botany, Polish Academy America, and have witnessed the of Sciences, Lubicz, Kraków, Poland. -

Recurrence of Stachybotrys Chartarum During Mycological And
Recurrence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed Caroline Lanier, Veronique Andre, Virginie Seguin, Natacha Heutte, Anne El Kaddoumi, Valérie Bouchart, Rachel Picquet, David Garon To cite this version: Caroline Lanier, Veronique Andre, Virginie Seguin, Natacha Heutte, Anne El Kaddoumi, et al.. Recur- rence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed. Annals of Agricultural and Environmental Medicine (AAEM), Institute of Agricultural Medicine in Lublin, 2012, 19 (1), pp.61-67. hal-02186772 HAL Id: hal-02186772 https://hal-normandie-univ.archives-ouvertes.fr/hal-02186772 Submitted on 13 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Annals of Agricultural and Environmental Medicine 2012, Vol 19, No 1, 61-67 ORIGINAL ARTICLE www.aaem.pl Recurrence of Stachybotrys chartarum during mycological and toxicological study of bioaerosols collected in a dairy cattle shed Caroline Lanier1, Véronique André1, Virginie Séguin1, Natacha Heutte1, Anne El Kaddoumi1, Valérie Bouchart2, Rachel Picquet2, David Garon1 1 Université de Caen Basse Normandie, Caen, France 2 Laboratoire Départemental Frank Duncombe, Conseil Général du Calvados, Saint Contest, France Lanier C, André V, Séguin V, Heutte N, Kaddoumi A, Bouchart V, Picquet R, Garon D. -

Culture Inventory
For queries, contact the SFA leader: John Dunbar - [email protected] Fungal collection Putative ID Count Ascomycota Incertae sedis 4 Ascomycota Incertae sedis 3 Pseudogymnoascus 1 Basidiomycota Incertae sedis 1 Basidiomycota Incertae sedis 1 Capnodiales 29 Cladosporium 27 Mycosphaerella 1 Penidiella 1 Chaetothyriales 2 Exophiala 2 Coniochaetales 75 Coniochaeta 56 Lecythophora 19 Diaporthales 1 Prosthecium sp 1 Dothideales 16 Aureobasidium 16 Dothideomycetes incertae sedis 3 Dothideomycetes incertae sedis 3 Entylomatales 1 Entyloma 1 Eurotiales 393 Arthrinium 2 Aspergillus 172 Eladia 2 Emericella 5 Eurotiales 2 Neosartorya 1 Paecilomyces 13 Penicillium 176 Talaromyces 16 Thermomyces 4 Exobasidiomycetes incertae sedis 7 Tilletiopsis 7 Filobasidiales 53 Cryptococcus 53 Fungi incertae sedis 13 Fungi incertae sedis 12 Veroneae 1 Glomerellales 1 Glomerella 1 Helotiales 34 Geomyces 32 Helotiales 1 Phialocephala 1 Hypocreales 338 Acremonium 20 Bionectria 15 Cosmospora 1 Cylindrocarpon 2 Fusarium 45 Gibberella 1 Hypocrea 12 Ilyonectria 13 Lecanicillium 5 Myrothecium 9 Nectria 1 Pochonia 29 Purpureocillium 3 Sporothrix 1 Stachybotrys 3 Stanjemonium 2 Tolypocladium 1 Tolypocladium 2 Trichocladium 2 Trichoderma 171 Incertae sedis 20 Oidiodendron 20 Mortierellales 97 Massarineae 2 Mortierella 92 Mortierellales 3 Mortiererallales 2 Mortierella 2 Mucorales 109 Absidia 4 Backusella 1 Gongronella 1 Mucor 25 RhiZopus 13 Umbelopsis 60 Zygorhynchus 5 Myrmecridium 2 Myrmecridium 2 Onygenales 4 Auxarthron 3 Myceliophthora 1 Pezizales 2 PeZiZales 1 TerfeZia 1 -

Sequencing Abstracts Msa Annual Meeting Berkeley, California 7-11 August 2016
M S A 2 0 1 6 SEQUENCING ABSTRACTS MSA ANNUAL MEETING BERKELEY, CALIFORNIA 7-11 AUGUST 2016 MSA Special Addresses Presidential Address Kerry O’Donnell MSA President 2015–2016 Who do you love? Karling Lecture Arturo Casadevall Johns Hopkins Bloomberg School of Public Health Thoughts on virulence, melanin and the rise of mammals Workshops Nomenclature UNITE Student Workshop on Professional Development Abstracts for Symposia, Contributed formats for downloading and using locally or in a Talks, and Poster Sessions arranged by range of applications (e.g. QIIME, Mothur, SCATA). 4. Analysis tools - UNITE provides variety of analysis last name of primary author. Presenting tools including, for example, massBLASTer for author in *bold. blasting hundreds of sequences in one batch, ITSx for detecting and extracting ITS1 and ITS2 regions of ITS 1. UNITE - Unified system for the DNA based sequences from environmental communities, or fungal species linked to the classification ATOSH for assigning your unknown sequences to *Abarenkov, Kessy (1), Kõljalg, Urmas (1,2), SHs. 5. Custom search functions and unique views to Nilsson, R. Henrik (3), Taylor, Andy F. S. (4), fungal barcode sequences - these include extended Larsson, Karl-Hnerik (5), UNITE Community (6) search filters (e.g. source, locality, habitat, traits) for 1.Natural History Museum, University of Tartu, sequences and SHs, interactive maps and graphs, and Vanemuise 46, Tartu 51014; 2.Institute of Ecology views to the largest unidentified sequence clusters and Earth Sciences, University of Tartu, Lai 40, Tartu formed by sequences from multiple independent 51005, Estonia; 3.Department of Biological and ecological studies, and for which no metadata Environmental Sciences, University of Gothenburg, currently exists. -

Morphological and Molecular Characterization of Alternaria Sect
Asian Journal of Biochemistry, Genetics and Molecular Biology 5(4): 30-41, 2020; Article no.AJBGMB.60766 ISSN: 2582-3698 Morphological and Molecular Characterization of Alternaria sect. Ulocladioides/Ulocladium Isolated from Citrus Fruits in Upper Egypt Youssuf A. Gherbawy1, Thanaa A. Maghraby1, Karima E. Abdel Fattah1 and Mohamed A. Hussein1* 1Botany and Microbiology Department, Faculty of Science, South Valley University, Qena 83523, Egypt. Authors’ contributions This work was carried out in collaboration among all authors. Author YAG designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Authors TAM and KEA managed the analyses of the study. Author MAH managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJBGMB/2020/v5i430138 Editor(s): (1) Dr. Arulselvan Palanisamy, Muthayammal College of Arts and Science, India. Reviewers: (1) S. Kannadhasan, Cheran College of Engineering, India. (2) J. Judith Vijaya, Loyola College, India. Complete Peer review History: http://www.sdiarticle4.com/review-history/60766 Received 20 July 2020 Accepted 26 September 2020 Original Research Article Published 20 October 2020 ABSTRACT Citrus is the most important crop in Upper Egypt. 150 infected samples were collected from citrus samples (Navel orange, Tangerine and Lemon) in Upper Egypt, 50 samples from each fruit. Twenty- two isolates representing three species of Alternaria belong to A. sect. Ulocladioides and A. sect. Ulocladium were isolated on dichloran chloramphenicol- peptone agar (DCPA) medium at 27°C. Tangerine samples were more contaminated with Alternaria followed by Navel orange and no Alternaria species appeared from Lemon samples. -

Biological Control in Greenhouse Systems
11 Jul 2001 14:56 AR AR138-06.tex AR138-06.SGM ARv2(2001/05/10) P1: GJB Annu. Rev. Phytopathol. 2001. 39:103–33 Copyright c 2001 by Annual Reviews. All rights reserved BIOLOGICAL CONTROL IN GREENHOUSE SYSTEMS Timothy C. Paulitz USDA-ARS Root Disease and Biocontrol Research Unit, 363 Johnson Hall, Washington State University, Pullman, Washington 99164-6430; e-mail: [email protected] Richard R. Belanger´ Departement´ de Phytologie, Universite´ Laval, Ste. Foy, Quebec,´ Canada G1K 7P9; e-mail: [email protected] Key Words biological control, Pythium root rot, powdery mildew, Pseudomonas, Trichoderma, Pseudozyma flocculosa ■ Abstract The controlled environment of greenhouses, the high value of the crops, and the limited number of registered fungicides offer a unique niche for the biological control of plant diseases. During the past ten years, over 80 biocontrol products have been marketed worldwide. A large percentage of these have been developed for green- house crops. Products to control soilborne pathogens such as Sclerotinia, Pythium, Rhizoctonia and Fusarium include Coniothyrium minitans, species of Gliocladium, Trichoderma, Streptomyces, and Bacillus, and nonpathogenic Fusarium. Products con- taining Trichoderma, Ampelomyces quisqualis, Bacillus, and Ulocladium are being developed to control the primary foliar diseases, Botrytis and powdery mildew. The development of Pseudomonas for the control of Pythium diseases in hydroponics and Pseudozyma flocculosa for the control of powdery mildew by two Canadian research programs is presented. In the future, biological control of diseases in greenhouses could predominate over chemical pesticides, in the same way that biological control of greenhouse insects predominates in the United Kingdom. The limitations in formu- lation, registration, and commercialization are discussed, along with suggested future by Ondokuz Mayis Universitesi on 09/18/14. -

HHE Report No. HETA-1999-0014-3094, Springdale
This Health Hazard Evaluation (HHE) report and any recommendations made herein are for the specific facility evaluated and may not be universally applicable. Any recommendations made are not to be considered as final statements of NIOSH policy or of any agency or individual involved. Additional HHE reports are available at http://www.cdc.gov/niosh/hhe/ Workplace Safety and Health Evaluation of Mold Contamination in a Hotel Before and After Remediation Kenneth Martinez, CIH, MSEE Douglas Trout, MD, MHS Kenneth Wallingford, MS, CIH Chandran Achutan, PhD Mitchell Singal, MD, MPH Health Hazard Evaluation Report HETA 1999-0014-3094 Springdale Best Western Hotel Cincinnati, Ohio November 2009 Department of Health and Human Services Centers for Disease Control and Prevention National Institute for Occupational Safety and Health The employer shall post a copy of this report for a period of 30 calendar days at or near the workplace(s) of affected employees. The employer shall take steps to insure that the posted determinations are not altered, defaced, or covered by other material during such period. [37 FR 23640, November 7, 1972, as amended at 45 FR 2653, January 14, 1980]. CONTENTS REPO R T Highlights of the NIOSH Health Hazard Evaluation .............ii Summary ............................................................................ iii Introduction ..........................................................................1 Background .........................................................................1 Methods ...............................................................................2