Novel Dematiaceous Hyphomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Unusual Haemoid Fungi: Ulocladium, As a Cause Of
Volume 2 Number 2 (June 2010) 95-97 CASE Report An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis Kaur R, Wadhwa A1,Gulati A2, Agrawal AK2 1Department of Microbiology. 2Department of Otorhinolaryngology, Maulana Azad Medical College, New Delhi, India. Received: April 2010, Accepted: May 2010. ABSTRACT Allergic fungal sinusitis (AFS) has been recognized as an important cause of chronic sinusitis commonly caused by Aspergillus spp. and various dematiaceous fungi like Bipolaris, Alternaria, Curvalaria, and etc. Ulocladium botrytis is a non pathogenic environmental dematiaceous fungi, which has been recently described as a human pathogen. Ulocladium has never been associated with allergic fungal sinusitis but it was identified as an etiological agent of AFS in a 35 year old immunocompetent female patient presenting with chronic nasal obstruction of several months duration to our hospital. The patient underwent FESS and the excised polyps revealed Ulocladium as the causative fungal agent. Keywords: Ulocladium, Phaeoid Fungi, Chronic Sinusitis. INTRODUCTION CASE REPORT Chronic allergic sinusitis is a common condition A 35 year old female patient presented to the ENT responsible for the development of nasal polyps, out patient department of the Lok Nayak Hospital, described as abnormal lesions that emanate from Delhi, with chronic nasal obstruction, excessive any portion of the nasal mucosa or Para nasal sneezing, nasal discharge and frontal headache since sinuses. They are commonly located in the middle several months. Nasal obstruction was of gradual meatus and ethmoid sinus and are present in onset, non progressive, more on the left side than 1-4% of the population (1). -
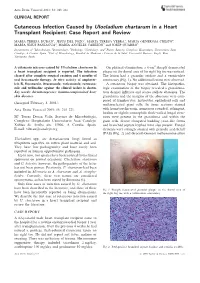
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

PERSOONIAL R Eflections
Persoonia 23, 2009: 177–208 www.persoonia.org doi:10.3767/003158509X482951 PERSOONIAL R eflections Editorial: Celebrating 50 years of Fungal Biodiversity Research The year 2009 represents the 50th anniversary of Persoonia as the message that without fungi as basal link in the food chain, an international journal of mycology. Since 2008, Persoonia is there will be no biodiversity at all. a full-colour, Open Access journal, and from 2009 onwards, will May the Fungi be with you! also appear in PubMed, which we believe will give our authors even more exposure than that presently achieved via the two Editors-in-Chief: independent online websites, www.IngentaConnect.com, and Prof. dr PW Crous www.persoonia.org. The enclosed free poster depicts the 50 CBS Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT most beautiful fungi published throughout the year. We hope Utrecht, The Netherlands. that the poster acts as further encouragement for students and mycologists to describe and help protect our planet’s fungal Dr ME Noordeloos biodiversity. As 2010 is the international year of biodiversity, we National Herbarium of the Netherlands, Leiden University urge you to prominently display this poster, and help distribute branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands. Book Reviews Mu«enko W, Majewski T, Ruszkiewicz- The Cryphonectriaceae include some Michalska M (eds). 2008. A preliminary of the most important tree pathogens checklist of micromycetes in Poland. in the world. Over the years I have Biodiversity of Poland, Vol. 9. Pp. personally helped collect populations 752; soft cover. Price 74 €. W. Szafer of some species in Africa and South Institute of Botany, Polish Academy America, and have witnessed the of Sciences, Lubicz, Kraków, Poland. -

Culture Inventory
For queries, contact the SFA leader: John Dunbar - [email protected] Fungal collection Putative ID Count Ascomycota Incertae sedis 4 Ascomycota Incertae sedis 3 Pseudogymnoascus 1 Basidiomycota Incertae sedis 1 Basidiomycota Incertae sedis 1 Capnodiales 29 Cladosporium 27 Mycosphaerella 1 Penidiella 1 Chaetothyriales 2 Exophiala 2 Coniochaetales 75 Coniochaeta 56 Lecythophora 19 Diaporthales 1 Prosthecium sp 1 Dothideales 16 Aureobasidium 16 Dothideomycetes incertae sedis 3 Dothideomycetes incertae sedis 3 Entylomatales 1 Entyloma 1 Eurotiales 393 Arthrinium 2 Aspergillus 172 Eladia 2 Emericella 5 Eurotiales 2 Neosartorya 1 Paecilomyces 13 Penicillium 176 Talaromyces 16 Thermomyces 4 Exobasidiomycetes incertae sedis 7 Tilletiopsis 7 Filobasidiales 53 Cryptococcus 53 Fungi incertae sedis 13 Fungi incertae sedis 12 Veroneae 1 Glomerellales 1 Glomerella 1 Helotiales 34 Geomyces 32 Helotiales 1 Phialocephala 1 Hypocreales 338 Acremonium 20 Bionectria 15 Cosmospora 1 Cylindrocarpon 2 Fusarium 45 Gibberella 1 Hypocrea 12 Ilyonectria 13 Lecanicillium 5 Myrothecium 9 Nectria 1 Pochonia 29 Purpureocillium 3 Sporothrix 1 Stachybotrys 3 Stanjemonium 2 Tolypocladium 1 Tolypocladium 2 Trichocladium 2 Trichoderma 171 Incertae sedis 20 Oidiodendron 20 Mortierellales 97 Massarineae 2 Mortierella 92 Mortierellales 3 Mortiererallales 2 Mortierella 2 Mucorales 109 Absidia 4 Backusella 1 Gongronella 1 Mucor 25 RhiZopus 13 Umbelopsis 60 Zygorhynchus 5 Myrmecridium 2 Myrmecridium 2 Onygenales 4 Auxarthron 3 Myceliophthora 1 Pezizales 2 PeZiZales 1 TerfeZia 1 -

Sequencing Abstracts Msa Annual Meeting Berkeley, California 7-11 August 2016
M S A 2 0 1 6 SEQUENCING ABSTRACTS MSA ANNUAL MEETING BERKELEY, CALIFORNIA 7-11 AUGUST 2016 MSA Special Addresses Presidential Address Kerry O’Donnell MSA President 2015–2016 Who do you love? Karling Lecture Arturo Casadevall Johns Hopkins Bloomberg School of Public Health Thoughts on virulence, melanin and the rise of mammals Workshops Nomenclature UNITE Student Workshop on Professional Development Abstracts for Symposia, Contributed formats for downloading and using locally or in a Talks, and Poster Sessions arranged by range of applications (e.g. QIIME, Mothur, SCATA). 4. Analysis tools - UNITE provides variety of analysis last name of primary author. Presenting tools including, for example, massBLASTer for author in *bold. blasting hundreds of sequences in one batch, ITSx for detecting and extracting ITS1 and ITS2 regions of ITS 1. UNITE - Unified system for the DNA based sequences from environmental communities, or fungal species linked to the classification ATOSH for assigning your unknown sequences to *Abarenkov, Kessy (1), Kõljalg, Urmas (1,2), SHs. 5. Custom search functions and unique views to Nilsson, R. Henrik (3), Taylor, Andy F. S. (4), fungal barcode sequences - these include extended Larsson, Karl-Hnerik (5), UNITE Community (6) search filters (e.g. source, locality, habitat, traits) for 1.Natural History Museum, University of Tartu, sequences and SHs, interactive maps and graphs, and Vanemuise 46, Tartu 51014; 2.Institute of Ecology views to the largest unidentified sequence clusters and Earth Sciences, University of Tartu, Lai 40, Tartu formed by sequences from multiple independent 51005, Estonia; 3.Department of Biological and ecological studies, and for which no metadata Environmental Sciences, University of Gothenburg, currently exists. -

Morphological and Molecular Characterization of Alternaria Sect
Asian Journal of Biochemistry, Genetics and Molecular Biology 5(4): 30-41, 2020; Article no.AJBGMB.60766 ISSN: 2582-3698 Morphological and Molecular Characterization of Alternaria sect. Ulocladioides/Ulocladium Isolated from Citrus Fruits in Upper Egypt Youssuf A. Gherbawy1, Thanaa A. Maghraby1, Karima E. Abdel Fattah1 and Mohamed A. Hussein1* 1Botany and Microbiology Department, Faculty of Science, South Valley University, Qena 83523, Egypt. Authors’ contributions This work was carried out in collaboration among all authors. Author YAG designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Authors TAM and KEA managed the analyses of the study. Author MAH managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJBGMB/2020/v5i430138 Editor(s): (1) Dr. Arulselvan Palanisamy, Muthayammal College of Arts and Science, India. Reviewers: (1) S. Kannadhasan, Cheran College of Engineering, India. (2) J. Judith Vijaya, Loyola College, India. Complete Peer review History: http://www.sdiarticle4.com/review-history/60766 Received 20 July 2020 Accepted 26 September 2020 Original Research Article Published 20 October 2020 ABSTRACT Citrus is the most important crop in Upper Egypt. 150 infected samples were collected from citrus samples (Navel orange, Tangerine and Lemon) in Upper Egypt, 50 samples from each fruit. Twenty- two isolates representing three species of Alternaria belong to A. sect. Ulocladioides and A. sect. Ulocladium were isolated on dichloran chloramphenicol- peptone agar (DCPA) medium at 27°C. Tangerine samples were more contaminated with Alternaria followed by Navel orange and no Alternaria species appeared from Lemon samples. -

Biological Control in Greenhouse Systems
11 Jul 2001 14:56 AR AR138-06.tex AR138-06.SGM ARv2(2001/05/10) P1: GJB Annu. Rev. Phytopathol. 2001. 39:103–33 Copyright c 2001 by Annual Reviews. All rights reserved BIOLOGICAL CONTROL IN GREENHOUSE SYSTEMS Timothy C. Paulitz USDA-ARS Root Disease and Biocontrol Research Unit, 363 Johnson Hall, Washington State University, Pullman, Washington 99164-6430; e-mail: [email protected] Richard R. Belanger´ Departement´ de Phytologie, Universite´ Laval, Ste. Foy, Quebec,´ Canada G1K 7P9; e-mail: [email protected] Key Words biological control, Pythium root rot, powdery mildew, Pseudomonas, Trichoderma, Pseudozyma flocculosa ■ Abstract The controlled environment of greenhouses, the high value of the crops, and the limited number of registered fungicides offer a unique niche for the biological control of plant diseases. During the past ten years, over 80 biocontrol products have been marketed worldwide. A large percentage of these have been developed for green- house crops. Products to control soilborne pathogens such as Sclerotinia, Pythium, Rhizoctonia and Fusarium include Coniothyrium minitans, species of Gliocladium, Trichoderma, Streptomyces, and Bacillus, and nonpathogenic Fusarium. Products con- taining Trichoderma, Ampelomyces quisqualis, Bacillus, and Ulocladium are being developed to control the primary foliar diseases, Botrytis and powdery mildew. The development of Pseudomonas for the control of Pythium diseases in hydroponics and Pseudozyma flocculosa for the control of powdery mildew by two Canadian research programs is presented. In the future, biological control of diseases in greenhouses could predominate over chemical pesticides, in the same way that biological control of greenhouse insects predominates in the United Kingdom. The limitations in formu- lation, registration, and commercialization are discussed, along with suggested future by Ondokuz Mayis Universitesi on 09/18/14. -

Fungal Glossary Spore Trap
Summary List of Fungi Included in this Glossary Report Alternaria sp. Ascospores Aspergillus sp. Basidiospores Chaetomium sp. Cladosporium sp. Curvularia sp. Drechslera, Bipolaris, and Exserohilum group Epicoccum sp. Memnoniella sp. Myxomycetes Penicillium sp. Pithomyces sp. Rusts Smuts Stachybotrys sp. Ulocladium sp. Eurofins EPK Built Environment Testing, LLC EMLab ID: 1014146, Page 1 of 23 Eurofins EMLab P&K 6000 Shoreline Ct, Ste 205, So. San Francisco, CA 94080 (866) 888-6653 Fax (623) 780-7695 www.emlab.com Alternaria sp. Mitosporic fungus. Hyphomycetes. Anamorphic Pleosporaceae. Distribution Ubiquitous; cosmopolitan. Approx. 40-50 species. Where Found Soil, dead organic debris, on food stuffs and textiles. Plant pathogen, most commonly on weakened plants. Mode of Dissemination Dry spore. Wind. Allergen Commonly recognized. Type I allergies (hay fever, asthma). Type III hypersensitivity pneumonitis: Woodworker's lung, Apple store hypersensitivity. May cross react with Ulocladium, Stemphylium, Phoma, others. Potential Opportunist of Pathogen Nasal lesions, subcutaneous lesions, nail infections; the majority of infections reported from persons with underlying disease or in those taking immunosuppressive drugs. Most species of Alternaria do not grow at 37oC. Potential Toxin Production A. alternata produces the antifungal alternariol. Other metabolites include AME (alternariol monomethylether), tenuazonic acid, and altertoxins (mutagenic). Growth Indoors On a variety of substrates. Aw=0.85-0.88 (minimum for various species) Industrial Uses Biocontrol of weeds and other plants. Other Comments One of the most common fungi worldwide. Characteristics: Growth/Culture Grows well on general fungal media. Colonies are dark olive green to brown, floccose to velvety (heavily sporulating). Colonies become pleomorphic over time, and lose the ability to sporulate with subsequent transfer. -

The Effect of Spaceflight on Growth of Ulocladium Chartarum Colonies on the International Space Station
The Effect of Spaceflight on Growth of Ulocladium chartarum Colonies on the International Space Station Ioana Gomoiu1*, Elias Chatzitheodoridis2, Sonia Vadrucci3, Isabelle Walther3 1 Institute of Biology Bucharest, Romanian Academy of Science, Bucharest, Romania, 2 School of Mining and Metallurgical Engineering, National Technical University of Athens, Athens, Greece, 3 Space Biology Group, ETH Zu¨rich, Zu¨rich, Switzerland Abstract The objectives of this 14 days experiment were to investigate the effect of spaceflight on the growth of Ulocladium chartarum, to study the viability of the aerial and submerged mycelium and to put in evidence changes at the cellular level. U. chartarum was chosen for the spaceflight experiment because it is well known to be involved in biodeterioration of organic and inorganic substrates covered with organic deposits and expected to be a possible contaminant in Spaceships. Colonies grown on the International Space Station (ISS) and on Earth were analysed post-flight. This study clearly indicates that U. chartarum is able to grow under spaceflight conditions developing, as a response, a complex colony morphotype never mentioned previously. We observed that spaceflight reduced the rate of growth of aerial mycelium, but stimulated the growth of submerged mycelium and of new microcolonies. In Spaceships and Space Stations U. chartarum and other fungal species could find a favourable environment to grow invasively unnoticed in the depth of surfaces containing very small amount of substrate, posing a risk factor for biodegradation of structural components, as well as a direct threat for crew health. The colony growth cycle of U. chartarum provides a useful eukaryotic system for the study of fungal growth under spaceflight conditions. -

Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit
Journal of Fungi Article Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit Francesco Aloi 1,2,†, Mario Riolo 1,3,4,† , Simona Marianna Sanzani 5, Annamaria Mincuzzi 6, Antonio Ippolito 6 , Ilenia Siciliano 7 , Antonella Pane 1,* , Maria Lodovica Gullino 7 and Santa Olga Cacciola 1,* 1 Department of Agriculture, Food and Environment, University of Catania, 95123 Catania, Italy; [email protected] (F.A.); [email protected] (M.R.) 2 Department of Agricultural, Food and Forest Sciences, University of Palermo, 90128 Palermo, Italy 3 Council for Agricultural Research and Agricultural Economy Analysis, Research Centre for Olive, Citrus and Tree Fruit–Rende CS (CREA- OFA), 87036 Rende, Italy 4 Department of Agricultural Science, Mediterranean University of Reggio Calabria, 89122 Reggio Calabria, Italy 5 CIHEAM Bari, Via Ceglie 9, 70010 Valenzano, Italy; [email protected] 6 Department of Soil, Plant, and Food Sciences, University of Bari Aldo Moro, Via Amendola 165/A, 70126 Bari, Italy; [email protected] (A.M.); [email protected] (A.I.) 7 Agroinnova—Centre of Competence for the Innovation in the Agro-Environmental Sector, University of Turin, 10095 Turin, Italy; [email protected] (I.S.); [email protected] (M.L.G.) * Correspondence: [email protected] (A.P.); [email protected] (S.O.C.) † These authors contributed equally to this work. Abstract: This study was aimed at identifying Alternaria species associated with heart rot disease of pomegranate fruit in southern Italy and characterizing their mycotoxigenic profile. A total of 42 Alternaria isolates were characterized. They were obtained from pomegranate fruits with symptoms Citation: Aloi, F.; Riolo, M.; Sanzani, of heart rot sampled in Apulia and Sicily and grouped into six distinct morphotypes based on S.M.; Mincuzzi, A.; Ippolito, A.; macro- and microscopic features. -

Proposed Generic Names for Dothideomycetes
Naming and outline of Dothideomycetes–2014 Nalin N. Wijayawardene1, 2, Pedro W. Crous3, Paul M. Kirk4, David L. Hawksworth4, 5, 6, Dongqin Dai1, 2, Eric Boehm7, Saranyaphat Boonmee1, 2, Uwe Braun8, Putarak Chomnunti1, 2, , Melvina J. D'souza1, 2, Paul Diederich9, Asha Dissanayake1, 2, 10, Mingkhuan Doilom1, 2, Francesco Doveri11, Singang Hongsanan1, 2, E.B. Gareth Jones12, 13, Johannes Z. Groenewald3, Ruvishika Jayawardena1, 2, 10, James D. Lawrey14, Yan Mei Li15, 16, Yong Xiang Liu17, Robert Lücking18, Hugo Madrid3, Dimuthu S. Manamgoda1, 2, Jutamart Monkai1, 2, Lucia Muggia19, 20, Matthew P. Nelsen18, 21, Ka-Lai Pang22, Rungtiwa Phookamsak1, 2, Indunil Senanayake1, 2, Carol A. Shearer23, Satinee Suetrong24, Kazuaki Tanaka25, Kasun M. Thambugala1, 2, 17, Saowanee Wikee1, 2, Hai-Xia Wu15, 16, Ying Zhang26, Begoña Aguirre-Hudson5, Siti A. Alias27, André Aptroot28, Ali H. Bahkali29, Jose L. Bezerra30, Jayarama D. Bhat1, 2, 31, Ekachai Chukeatirote1, 2, Cécile Gueidan5, Kazuyuki Hirayama25, G. Sybren De Hoog3, Ji Chuan Kang32, Kerry Knudsen33, Wen Jing Li1, 2, Xinghong Li10, ZouYi Liu17, Ausana Mapook1, 2, Eric H.C. McKenzie34, Andrew N. Miller35, Peter E. Mortimer36, 37, Dhanushka Nadeeshan1, 2, Alan J.L. Phillips38, Huzefa A. Raja39, Christian Scheuer19, Felix Schumm40, Joanne E. Taylor41, Qing Tian1, 2, Saowaluck Tibpromma1, 2, Yong Wang42, Jianchu Xu3, 4, Jiye Yan10, Supalak Yacharoen1, 2, Min Zhang15, 16, Joyce Woudenberg3 and K. D. Hyde1, 2, 37, 38 1Institute of Excellence in Fungal Research and 2School of Science, Mae Fah Luang University, -

Mating-Type Genes of the Anamorphic Fungus Ulocladium Botrytis Affect
www.nature.com/scientificreports OPEN Mating-type genes of the anamorphic fungus Ulocladium botrytis afect both asexual Received: 20 December 2016 Accepted: 13 July 2017 sporulation and sexual Published: xx xx xxxx reproduction Qun Wang1, Shi Wang1, Chen Lin Xiong1, Timothy Y. James2 & Xiu Guo Zhang 1 Ulocladium was thought to be a strictly asexual genus of flamentous fungi. However, Ulocladium strains were shown to possess both MAT1-1-1 and MAT1-2-1 genes as observed in homothallic flamentous Ascomycetes. Here, we demonstrate that the U. botrytis MAT genes play essential roles for controlling asexual traits (conidial size and number). Using reciprocal genetic transformation, we demonstrate that MAT genes from the related heterothallic species Cochliobolus heterostrophus can also infuence U. botrytis colony growth, conidial number and size, and have a strong efect on the range of the number of septa/conidium. Moreover, U. botrytis MAT genes can also afect similar aspects of asexual reproduction when expressed in C. heterostrophus. Heterologous complementation using C. heterostrophus MAT genes shows that they have lost the ability to regulate sexual reproduction in U. botrytis, under the conditions we employed, while the reciprocal heterologous complementation demonstrates that U. botrytis MAT genes have the ability to partially induce sexual reproduction in C. heterostrophus. Thus, the genetic backgrounds of C. heterostrophus and U. botrytis play signifcant roles in determining the function of MAT genes on sexual reproduction in these two fungi species. These data further support the role of MAT genes in controlling asexual growth in flamentous Ascomycetes but also confrm that heterothallic and homothallic Dothideomycete fungi can be interconverted by the exchange of MAT genes.