Current Knowledge on the Fungal Degradation Abilities Profiled Through Biodeteriorative Plate Essays
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Evaluation of Rrna Targeted in Situ Probes and Phylogenetic Relationships of Freshwater Fungi
Development and evaluation of rRNA targeted in situ probes and phylogenetic relationships of freshwater fungi vorgelegt von Diplom-Biologin Christiane Baschien aus Berlin Von der Fakultät III - Prozesswissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktorin der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. sc. techn. Lutz-Günter Fleischer Berichter: Prof. Dr. rer. nat. Ulrich Szewzyk Berichter: Prof. Dr. rer. nat. Felix Bärlocher Berichter: Dr. habil. Werner Manz Tag der wissenschaftlichen Aussprache: 19.05.2003 Berlin 2003 D83 Table of contents INTRODUCTION ..................................................................................................................................... 1 MATERIAL AND METHODS .................................................................................................................. 8 1. Used organisms ............................................................................................................................. 8 2. Media, culture conditions, maintenance of cultures and harvest procedure.................................. 9 2.1. Culture media........................................................................................................................... 9 2.2. Culture conditions .................................................................................................................. 10 2.3. Maintenance of cultures.........................................................................................................10 -

Fungal Communities in Archives: Assessment Strategies and Impact on Paper Con- Servation and Human Health Fungal
Ana Catarina Martiniano da Silva Pinheiro Licenciada em Conservação e Restauro pela Universidade Nova de Lisboa Licenciada em Ciências Farmacêuticas pela Universidade de Lisboa [Nome completo do autor] [Nome completo do autor] [Habilitações Académicas] Fungal Communities in Archives: Assessment Strategies and Impact on Paper [Nome completo do autor] [Habilitações Académicas] Conservation and Human Health Dissertação para obtenção do Grau de Doutor em [Habilitações Académicas] Ciências[Nome da completoConservação do autor] pela Universidade Nova de Lisboa, Faculdade de Ciências e Tecnologia [NomeDissertação complet parao obtençãodo autor] do[Habilitações Grau de Mestre Académicas] em [Engenharia Informática] Orient ador: Doutora Filomena Macedo Dinis, Professor Auxiliar com Nomeação De- finitiva, DCR, FCT-UNL Co-orientadores:[Nome completo Doutora doLaura autor] Rosado, [Habilitações Instituto Nacional Académicas] de Saúde Doutor Ricardo Jorge I.P. Doutora Valme Jurado, Instituto de Recursos Naturales y Agrobiología, CSIC [Nome completo do autor] [Habilitações Académicas] Júri Presidente Prof. Doutor Fernando Pina Arguentes Doutor António Manuel Santos Carriço Portugal, Professor Auxiliar Doutor Alan Phillips, Investigador Vogais Doutora Maria Inês Durão de Carvalho Cordeiro, Directora da Biblioteca Nacional Doutora Susana Marta Lopes Almeida, Investigadora Auxiliar iii October, 2014 Fungal Communities in Archives: Assessment Strategies and Impact on Paper Con- servation and Human Health Fungal Copyright © Ana Catarina Martiniano da Silva -

Review of Oxepine-Pyrimidinone-Ketopiperazine Type Nonribosomal Peptides
H OH metabolites OH Review Review of Oxepine-Pyrimidinone-Ketopiperazine Type Nonribosomal Peptides Yaojie Guo , Jens C. Frisvad and Thomas O. Larsen * Department of Biotechnology and Biomedicine, Technical University of Denmark, Søltofts Plads, Building 221, DK-2800 Kgs. Lyngby, Denmark; [email protected] (Y.G.); [email protected] (J.C.F.) * Correspondence: [email protected]; Tel.: +45-4525-2632 Received: 12 May 2020; Accepted: 8 June 2020; Published: 15 June 2020 Abstract: Recently, a rare class of nonribosomal peptides (NRPs) bearing a unique Oxepine-Pyrimidinone-Ketopiperazine (OPK) scaffold has been exclusively isolated from fungal sources. Based on the number of rings and conjugation systems on the backbone, it can be further categorized into three types A, B, and C. These compounds have been applied to various bioassays, and some have exhibited promising bioactivities like antifungal activity against phytopathogenic fungi and transcriptional activation on liver X receptor α. This review summarizes all the research related to natural OPK NRPs, including their biological sources, chemical structures, bioassays, as well as proposed biosynthetic mechanisms from 1988 to March 2020. The taxonomy of the fungal sources and chirality-related issues of these products are also discussed. Keywords: oxepine; nonribosomal peptides; bioactivity; biosynthesis; fungi; Aspergillus 1. Introduction Nonribosomal peptides (NRPs), mostly found in bacteria and fungi, are a class of peptidyl secondary metabolites biosynthesized by large modularly organized multienzyme complexes named nonribosomal peptide synthetases (NRPSs) [1]. These products are amongst the most structurally diverse secondary metabolites in nature; they exhibit a broad range of activities, which have been exploited in treatments such as the immunosuppressant cyclosporine A and the antibiotic daptomycin [2,3]. -

43-09-405 and 407 Final Combine
Pak. J. Bot ., 44(6): 2093-2102, 2012. A NEW SPECIES OF TIAROSPORELLA AZADARICHTA AND NEW FUNGAL RECORDS ON AZADIRACHTA INDICA FROM PAKISTAN SYED QAISER ABBAS 1, TEHREEMA IFTIKHAR 1* , MUBASHIR NIAZ 1, IFTIKHAR ALI 1, NABILA IFTIKHAR 1 AND ALIA ABBAS 2 1Department of Botany, Government College University, Alama Iqbal Road, Faisalabad, Pakistan 2Department of Botany, Federal Urdu University, Karachi, Pakistan *Corresponding author’s e-mail: [email protected] Abstract A new species of Tiarosporella azadarichta has been described on Azadirachta indica and some new fungal records viz ., Diplozythiella bambusina , Ulocladium chartarum , Cladosporium nigrellum, Cladosporium oxysporum. Didymostilbe coffeae , Muellerella pygmaea, Lasiodiplodia paraphysaria, Monochaetinula terminalae, Trimmatostroma sp., and Epidermophyton floccosum are reported on Azadirachta indica for the first time from Pakistan. Introduction reason a detailed survey of the plant for fungi was under taken in this study. Azadirachta indica (as Azadirachta Juss, Melia azadirachta ) belong to the family Meliaceae. Neem is its Materials and Methods common name. It is important due to its commercial and medicinal value. Azadirachta indica is also an important Samples of Azadirachta indica were collected from plant for its potential of anti-fungal, anti-bacterial and the different areas of District Faisalabad and Gojra. The insecticidal activity. Decline of trees due to fungi are different areas were G.C. University. Faisalabad, increasing tremendously in Pakistan especially in Punjab Agriculture University Faisalabad, Gutwala forest (park) and Sindh (Javed et al ., 2004; Khanzada et al ., 2011; Fateh Faisalabad, Tandlianwala and Gojra City. Methods and et al., 2011; Rheman et al., 2011; Farooq et al., 2011). materials are the same as described Abbas et al ., (2010). -

Entomopathogenic Fungi and Bacteria in a Veterinary Perspective
biology Review Entomopathogenic Fungi and Bacteria in a Veterinary Perspective Valentina Virginia Ebani 1,2,* and Francesca Mancianti 1,2 1 Department of Veterinary Sciences, University of Pisa, viale delle Piagge 2, 56124 Pisa, Italy; [email protected] 2 Interdepartmental Research Center “Nutraceuticals and Food for Health”, University of Pisa, via del Borghetto 80, 56124 Pisa, Italy * Correspondence: [email protected]; Tel.: +39-050-221-6968 Simple Summary: Several fungal species are well suited to control arthropods, being able to cause epizootic infection among them and most of them infect their host by direct penetration through the arthropod’s tegument. Most of organisms are related to the biological control of crop pests, but, more recently, have been applied to combat some livestock ectoparasites. Among the entomopathogenic bacteria, Bacillus thuringiensis, innocuous for humans, animals, and plants and isolated from different environments, showed the most relevant activity against arthropods. Its entomopathogenic property is related to the production of highly biodegradable proteins. Entomopathogenic fungi and bacteria are usually employed against agricultural pests, and some studies have focused on their use to control animal arthropods. However, risks of infections in animals and humans are possible; thus, further studies about their activity are necessary. Abstract: The present study aimed to review the papers dealing with the biological activity of fungi and bacteria against some mites and ticks of veterinary interest. In particular, the attention was turned to the research regarding acarid species, Dermanyssus gallinae and Psoroptes sp., which are the cause of severe threat in farm animals and, regarding ticks, also pets. -

An Unusual Haemoid Fungi: Ulocladium, As a Cause Of
Volume 2 Number 2 (June 2010) 95-97 CASE Report An unusual phaeoid fungi: Ulocladium, as a cause of chronic allergic fungal sinusitis Kaur R, Wadhwa A1,Gulati A2, Agrawal AK2 1Department of Microbiology. 2Department of Otorhinolaryngology, Maulana Azad Medical College, New Delhi, India. Received: April 2010, Accepted: May 2010. ABSTRACT Allergic fungal sinusitis (AFS) has been recognized as an important cause of chronic sinusitis commonly caused by Aspergillus spp. and various dematiaceous fungi like Bipolaris, Alternaria, Curvalaria, and etc. Ulocladium botrytis is a non pathogenic environmental dematiaceous fungi, which has been recently described as a human pathogen. Ulocladium has never been associated with allergic fungal sinusitis but it was identified as an etiological agent of AFS in a 35 year old immunocompetent female patient presenting with chronic nasal obstruction of several months duration to our hospital. The patient underwent FESS and the excised polyps revealed Ulocladium as the causative fungal agent. Keywords: Ulocladium, Phaeoid Fungi, Chronic Sinusitis. INTRODUCTION CASE REPORT Chronic allergic sinusitis is a common condition A 35 year old female patient presented to the ENT responsible for the development of nasal polyps, out patient department of the Lok Nayak Hospital, described as abnormal lesions that emanate from Delhi, with chronic nasal obstruction, excessive any portion of the nasal mucosa or Para nasal sneezing, nasal discharge and frontal headache since sinuses. They are commonly located in the middle several months. Nasal obstruction was of gradual meatus and ethmoid sinus and are present in onset, non progressive, more on the left side than 1-4% of the population (1). -

De Novo Assembly and Genome Analyses of the Marine-Derived
De Novo Assembly and Genome Analyses of the Marine-Derived Scopulariopsis brevicaulis Strain LF580 Unravels Life-Style Traits and Anticancerous Scopularide Biosynthetic Gene Cluster Abhishek Kumar, Bernard Henrissat, Mikko Arvas, Muhammad Fahad Syed, Nils Thieme, J. Philipp Benz, Jens Laurids Sorensen, Eric Record, Stefanie Poeggeler, Frank Kempken To cite this version: Abhishek Kumar, Bernard Henrissat, Mikko Arvas, Muhammad Fahad Syed, Nils Thieme, et al.. De Novo Assembly and Genome Analyses of the Marine-Derived Scopulariopsis brevicaulis Strain LF580 Unravels Life-Style Traits and Anticancerous Scopularide Biosynthetic Gene Cluster. PLoS ONE, Public Library of Science, 2015, 10 (10), 10.1371/journal.pone.0140398. hal-01439026 HAL Id: hal-01439026 https://hal.archives-ouvertes.fr/hal-01439026 Submitted on 17 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License RESEARCH ARTICLE De Novo Assembly and Genome Analyses of the Marine-Derived Scopulariopsis brevicaulis Strain -

Aspergillus Tubingensis Causes Leaf Spot of Cotton (Gossypium Hirsutum L.) in Pakistan
Phyton, International Journal of Experimental Botany DOI: 10.32604/phyton.2020.08010 Article Aspergillus tubingensis Causes Leaf Spot of Cotton (Gossypium hirsutum L.) in Pakistan Maria Khizar1, Urooj Haroon1, Musrat Ali1, Samiah Arif2, Iftikhar Hussain Shah2, Hassan Javed Chaudhary1 and Muhammad Farooq Hussain Munis1,* 1Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan 2Department of Plant Sciences, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China *Corresponding Author: Muhammad Farooq Hussain Munis. Email: [email protected] Received: 20 July 2019; Accepted: 12 October 2019 Abstract: Cotton (Gossypium hirsutum L.) is a key fiber crop of great commercial importance. Numerous phytopathogens decimate crop production by causing various diseases. During July-August 2018, leaf spot symptoms were recurrently observed on cotton leaves in Rahim Yar Khan, Pakistan and adjacent areas. Infected leaf samples were collected and plated on potato dextrose agar (PDA) media. Causal agent of cotton leaf spot was isolated, characterized and identified as Aspergillus tubingensis based on morphological and microscopic observations. Conclusive identification of pathogen was done on the comparative molecular analysis of CaM and β-tubulin gene sequences. BLAST analysis of both sequenced genes showed 99% similarity with A. tubingensis. Koch’s postulates were followed to confirm the pathogenicity of the isolated fungus. Healthy plants were inoculated with fungus and similar disease symptoms were observed. Fungus was re-isolated and identified to be identical to the inoculated fungus. To our knowledge, this is the first report describing the involvement of A. tubingensis in causing leaf spot disease of cotton in Pakistan and around the world. -

Isolation of Scopulariopsis Brevicaulis from Wistar Rats
Etlik Vet Mikrobiyol Derg, 2020; 31 (2): 196-200 Case Report doi: https://doi.org/10.35864/evmd.768818 Olgu Sunumu Case report: Isolation of Scopulariopsis brevicaulis from Wistar Rats Özlem Şahan Yapıcıer1* , Mehmet Kaya2 , Zeki Erol3 , Dilek Öztürk4 1,2,4 Faculty of Veterinary Medicine, Mehmet Akif Ersoy University, Department of Microbiology, Burdur, TURKEY 3 Mehmet Akif Ersoy University, Experimental Animal Production and Experimental Research Center, Burdur, TURKEY Geliş Tarihi / Received: 13.07.2020, Kabul tarihi / Accepted: 07.12.2020 Abstract: Scopulariopsis brevicaulis is a saprophytic fungus that has wide geographic distribution. This study de- scribes a case of hair loss and skin lesions observed in male and female Wistar rats due to Scopulariopsis brevicaulis infection in Turkey. Skin scrapings and hair samples from three male and two female rats were provided by the Experimental Animal Production and Experimental Research Center of Mehmet Akif Ersoy University to the Faculty of Veterinary Medicine, Department of Microbiology Laboratory in Burdur for analysis in July 2019. Microbiological methods were used for species identification andScopulariopsis brevicaulis was isolated from all of the samples. The rats completely recovered without treatment and had no recurrence of clinical signs at one month post-sampling. This study is the first report ofS. brevicaulis causing an infection in Wistar rats in Turkey. Keywords: Laboratory animals, mycological examination, rats, saprophyte, Scopulariopsis sp Olgu sunumu: Wistar Ratlarından Scopulariopsis brevicularis izolasyonu Özet: Scopulariopsis brevicaulis, geniş coğrafi dağılımı olan saprofitik bir mantardır. Bu olgu, Türkiye’deki erkek ve dişi Wistar ratlarında Scopulariopsis brevicaulis infeksiyonuna bağlı olarak gözlenen tüy kaybı ve deri lezyonlarını tanımlamaktadır. -

Patents on Endophytic Fungi Related to Secondary Metabolites and Biotransformation Applications
Journal of Fungi Review Patents on Endophytic Fungi Related to Secondary Metabolites and Biotransformation Applications Daniel Torres-Mendoza 1,2 , Humberto E. Ortega 1,3 and Luis Cubilla-Rios 1,* 1 Laboratory of Tropical Bioorganic Chemistry, Faculty of Natural, Exact Sciences and Technology, University of Panama, Panama 0824, Panama; [email protected] (D.T.-M.); [email protected] (H.E.O.) 2 Vicerrectoría de Investigación y Postgrado, University of Panama, Panama 0824, Panama 3 Department of Organic Chemistry, Faculty of Natural, Exact Sciences and Technology, University of Panama, Panama 0824, Panama * Correspondence: [email protected]; Tel.: +507-6676-5824 Received: 31 March 2020; Accepted: 29 April 2020; Published: 1 May 2020 Abstract: Endophytic fungi are an important group of microorganisms and one of the least studied. They enhance their host’s resistance against abiotic stress, disease, insects, pathogens and mammalian herbivores by producing secondary metabolites with a wide spectrum of biological activity. Therefore, they could be an alternative source of secondary metabolites for applications in medicine, pharmacy and agriculture. In this review, we analyzed patents related to the production of secondary metabolites and biotransformation processes through endophytic fungi and their fields of application. Weexamined 245 patents (224 related to secondary metabolite production and 21 for biotransformation). The most patented fungi in the development of these applications belong to the Aspergillus, Fusarium, Trichoderma, Penicillium, and Phomopsis genera and cover uses in the biomedicine, agriculture, food, and biotechnology industries. Keywords: endophytic fungi; patents; secondary metabolites; biotransformation; biological activity 1. Introduction The term endophyte refers to any organism (bacteria or fungi) that lives in the internal tissues of a host. -
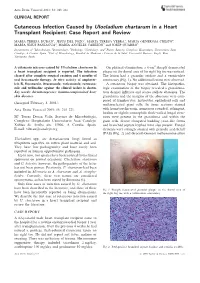
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Identification of Fungi Storage Types by Sequencing Method
Zh. T. Abdrassulova et al /J. Pharm. Sci. & Res. Vol. 10(3), 2018, 689-692 Identification of Fungi Storage Types by Sequencing Method Zh. T. Abdrassulova, A. M. Rakhmetova*, G. A. Tussupbekova#, Al-Farabi Kazakh national university, 050040, Republic of Kazakhstan, Almaty, al-Farabi Ave., 71 *Karaganda State University named after the academician E.A. Buketov #A;-Farabi Kazakh National University, 050040, Republic of Kazakhstan, Almaty, al0Farabi Ave, 71 E. M. Imanova, Kazakh State Women’s Teacher Training University, 050000, Republic of Kazakhstan, Almaty, Aiteke bi Str., 99 M. S. Agadieva, R. N. Bissalyyeva Aktobe regional governmental university named after K.Zhubanov, 030000, Republic of Kazakhstan, Аktobe, A. Moldagulova avenue, 34 Abstract With the use of classical identification methods, which imply the identification of fungi by cultural and morphological features, they may not be reliable. With the development of modern molecular methods, it became possible to quickly and accurately determine the species and race of the fungus. The purpose of this work was to study bioecology and refine the species composition of fungi of the genus Aspergillus, Penicillium on seeds of cereal crops. The article presents materials of scientific research on morphological and molecular genetic peculiarities of storage fungi, affecting seeds of grain crops. Particular attention is paid to the fungi that develop in the stored grain. The seeds of cereals (Triticum aestivum L., Avena sativa L., Hordeum vulgare L., Zea mays L., Oryza sativa L., Sorghum vulgare Pers., Panicum miliaceum L.) were collected from the granaries of five districts (Talgar, Iliysky, Karasai, Zhambul, Panfilov) of the Almaty region. The pathogens of diseases of fungal etiology were found from the genera Penicillium, Aspergillus influencing the safety, quality and safety of the grain.