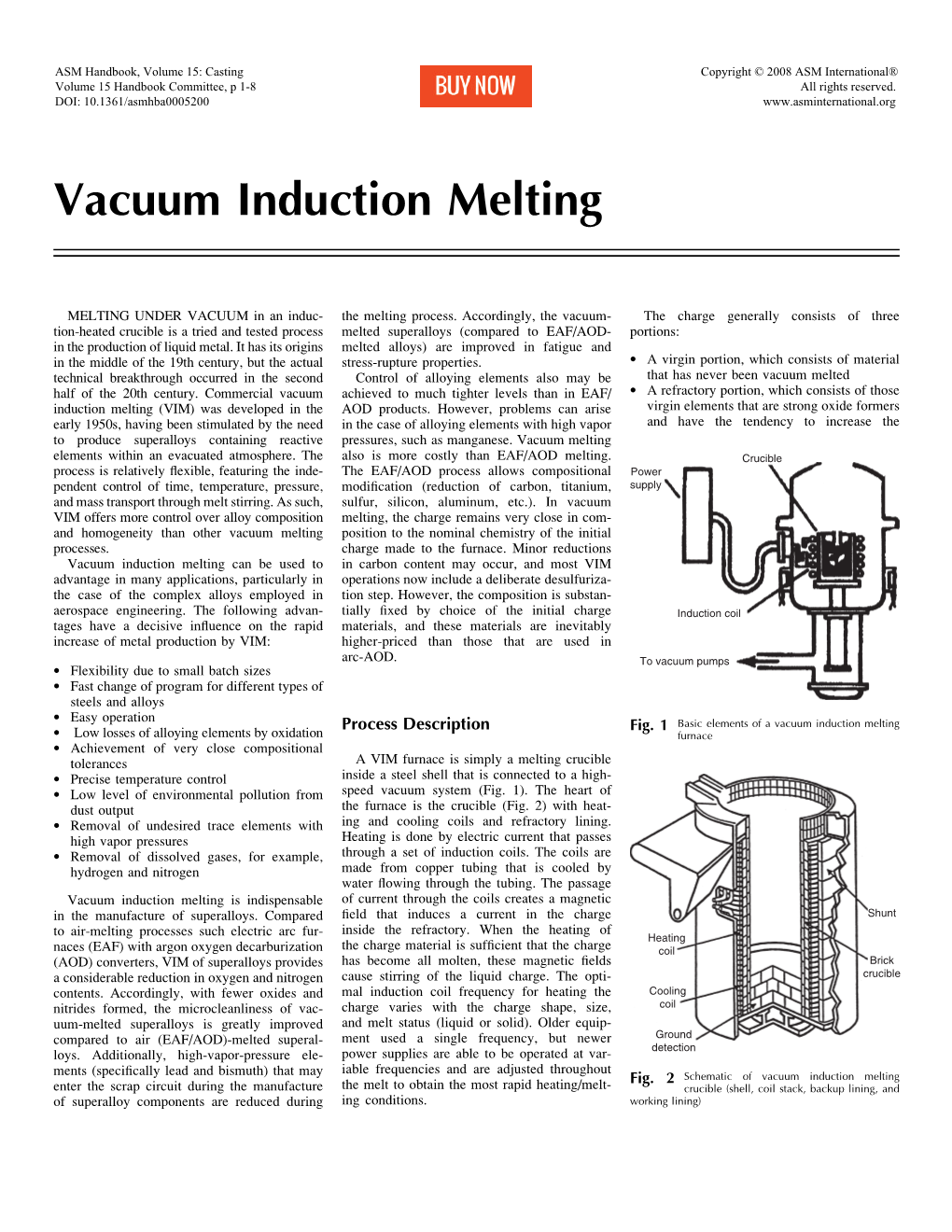
Vacuum Induction Melting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Parallel Oxidation of Carbon and Chromium
THE PARALLEL OXIDATION OF CARBON AND CHROMIUM IN LIQUID IRON (16oo0 c) by LYALL FRANKLIN BARNHARDT B. Sc. Queen's University (1947) Submitted in partial fullfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY at the Massachusetts Institute of Technology 1965 Signature of Author Department of Metallurgy Signature of Professor in Charge of Research jE Signature of Chairman of - A ii Departmental Committee on Graduate Students VI 38 ABSTRACT THE PARALLEL OXIDATION OF CARBON AND CHROMIUM IN LIQUID IRON (16000c) by LYALL FRANKLIN BARNHARDT Submitted to the Department of Metallurgy in April, 1965, in partial fulfillment of the requirements for the Degree of Doctor of Philosophy. The path followed by carbon and chromium concentrations in liquid iron during oxidation was investigated experimentally by blowing gaseous oxygen on to slag-free iron - chromium - carbon melts at 16004C in an induction furnace. The carbon oxidation reaction occurred at the surface of the melt, with an initial constant rate of decarburization which was dependent on the rate of oxygen input. The mechanism of this reaction is expressed in the chemical equation: C + 1/2 02 (g) = CO (g). During the initial stage, which was characterized by a constant rate of decarburization, carbon oxidized preferentially with no loss of chromium. Beyond this stage, the rate of carbon loss declined rapidly and the oxidation of iron and chromium occurred. The entry of argon into the furnace atmosphere, during the oxygen blow, lowered the partial pressure of carbon monoxide at the metal surface. This allowed the carbon concentration of the melt to drop far below the equilibrium iii carbon content for one atmosphere pressure of carbon monoxide. -

On a Mathematical Model for Case Hardening of Steel
On a mathematical model for case hardening of steel vorgelegt von Dott.ssa Lucia Panizzi Von der Fakultät II ‚ Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften Dr. rer. nat. sowie der Classe di Scienze der Scuola Normale Superiore di Pisa als Diploma di Perfezionamento in Matematica per la Tecnologia e l’Industria genehmigte Dissertation Promotionsausschuss: Berichter/Gutachter: Prof. A. Fasano (Univerisità di Firenze) Berichter/Gutachter: Prof. D. Hömberg (Technische Universität Berlin) Gutachter: Prof. L. Formaggia (Politecnico di Milano) Gutachter: Prof. M. Primicerio (Università di Firenze) Gutachter: Prof. F. Tröltzsch (Technische Universität Berlin) Gutachter: Prof. P. Wittbold (Technische Universität Berlin) Tag der wissenschaftlichen Aussprache: 05.03.2010 Berlin 2010 D83 i Eidesstattliche Versicherung Hiermit erkläre ich, dass die vorliegende Dissertation: On a mathematical model for case hardening of steel selbständig verfasst wurde. Die benutzten Hilfsmittel und Quellen wurden von mir angegeben, weitere wurden nicht verwendet. ii Erklärung Hiermit erkläre ich, dass die Anmeldung meiner Promotionsabsicht früher nicht bei einer anderen Hochschule oder einer anderen Fakultät beantragt wurde. Teile meiner Dissertation sind darüber hinaus schon veröffentlich worden, welche im Folgenden aufgelistet sind: • D. Hömberg, A. Fasano, L. Panizzi A mathematical model for case hardening of steel. angenommen zur Veröffentlichung in "Mathematical Models and Methods in Applied Sciences" (M3AS), 2009. • P. Krejčí, L. Panizzi. Regularity and uniqueness in quasilinear parabolic systems. angenommen zur Veröffentlichung in "Applications of Mathematics", 2009. iii Compendio Nonostante la disponibilità di numerosi nuovi materiali, l’acciaio rimane il ma- teriale di base della moderna società industriale. L’uso dell’ acciaio con peculiari caratteristiche (durezza, resistenza all’uso, malleabilità etc.) è perciò assai diffuso in molti settori della tecnica. -

Böhler Edelstahl - General Information
Content . Böhler Edelstahl - General Information . Production Possibilities & New Additive Manufacturing . Product Portfolio Böhler Edelstahl - General Information MISSION We develop, produce and supply high-speed steels, tool steels and special materials for our worldwide customers to offer them optimal solutions. BÖHLER Edelstahl at a glance Worldwide 2016 Production 79 €622 137,000 points of sale million revenue tonnes Advanced 2114 production facilities employees Quality & technology Global 250 LEADER steel grades 100% recyclable Sales revenue by region (FY 2016) 1% 9% 12% Europe America Asia Rest of world 78% Sales revenue by business area (FY 2016) 11% Special materials 41% 21% Tool steel High speed steels Other 27% Business area revenue special materials Automotive Other Engineering 4% 11% 11% Special Oil & Gas, materials CPI High speed Power-Gen steel 21% 41% 43% 15% 27% Tool steel 27% Aerospace Production Possibilities BÖHLER EDELSTAHL Integrated plant configuration Whole melting and re-melting operations at one site Whole hot forming operations at one site Whole heat treatment operations at one site Whole ND-Testing and finishing operations at one site Standard mechanical, metallographic, chemical tests at one site Quality starts here Primary metallurgy EAF/50 t Electric arc furnace VIM Vacuum induction furnace AOD Argon oxygen decarburization VID/14 t Vacuum induction furnace REMELTING Remelting process for high- purity metallurgical materials • ESR • PESR • VAR FORGING TECHNOLOGY 5,200 t forging press Open die forge Bar steel -

UNDERSTANDING and MEASURING DECARBURIZATION Understanding the Forces Behind Decarburization Is the First Step Toward Minimizing Its Detrimental Effects
22 UNDERSTANDING AND MEASURING DECARBURIZATION Understanding the forces behind decarburization is the first step toward minimizing its detrimental effects. George F. Vander Voort, FASM*, Struers Inc. (Consultant), Cleveland ecarburization is detrimental to crostructure of carbon contents close to the maximum depth of decarburization the wear life and fatigue life of the core may be difficult to discern. The is established. Because the carbon dif- steel heat-treated components. MAD determined by hardness traverse fusion rate increases with temperature ADVANCED MATERIALS & PROCESSES | FEBRUARY 2015 2015 FEBRUARY | & PROCESSES MATERIALS ADVANCED D This article explores some factors that may be slightly shallower than that de- when the structure is fully austenitic, cause decarburization while concentrat- termined by quantitative carbon analysis MAD also increases as temperature rises ing on its measurement. In most produc- with the electron microprobe. This is es- above the Ac3. For temperatures in the tion tests, light microscopes are used to pecially true when the bulk carbon con- two-phase region, between the Ac1 and scan the surface of a polished and etched tent exceeds about 0.45 wt%, as the rela- Ac3, the process is more complex. Carbon cross-section to find what appears to be tionship between carbon in the austenite diffusion rates in ferrite and austenite the greatest depth of total carbon loss before quenching to form martensite and are different, and are influenced by both (free-ferrite depth, or FFD) and the great- the as-quenched hardness loses its linear temperature and composition. est depth of combined FFD and partial nature above this carbon level. Decarburization is a serious prob- loss of carbon to determine the maxi- lem because surface properties are infe- mum affected depth (MAD). -

Method for Boriding of Coatings Using High Speed
(19) & (11) EP 2 222 898 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C25D 9/08 (2006.01) C23C 28/00 (2006.01) 08.06.2011 Bulletin 2011/23 C25D 3/66 (2006.01) C25D 9/04 (2006.01) (21) Application number: 08848442.3 (86) International application number: PCT/EP2008/065065 (22) Date of filing: 06.11.2008 (87) International publication number: WO 2009/060033 (14.05.2009 Gazette 2009/20) (54) METHOD FOR BORIDING OF COATINGS USING HIGH SPEED ELECTROLYTIC PROCESS VERFAHREN ZUM BORIEREN VON BESCHICHTUNGEN MIT HILFE EINES SCHNELLEN ELEKTROLYTISCHEN VERFAHRENS PROCÉDÉ DE BORURATION DE REVÊTEMENTS À L’AIDE D’UN TRAITEMENT ÉLECTROLYTIQUE HAUTE VITESSE (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Ürgen, Mustafa K. HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Istanbul (TR) RO SE SI SK TR • Timur, Servet I. Istanbul (TR) (30) Priority: 09.11.2007 EP 07120419 • Kazmanli, Kürsat M. Istanbul (TR) (43) Date of publication of application: • Kartal, Güldem 01.09.2010 Bulletin 2010/35 Istanbul (TR) (73) Proprietors: (74) Representative: Sevinç, Erkan • Ürgen, Mustafa K. Istanbul Patent & Trademark Consultancy Ltd. Istanbul (TR) Plaza-33, Büyükdere cad. No: 33/16 • Timur, Servet I. Sisli Istanbul (TR) 34381 Istanbul (TR) • Kazmanli, Kürsat M. Istanbul (TR) (56) References cited: • Kartal, Güldem DE-A1- 1 796 215 GB-A- 1 422 859 Istanbul (TR) JP-A- 5 161 090 US-A- 3 824 134 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

An Introduction to Nitriding
01_Nitriding.qxd 9/30/03 9:58 AM Page 1 © 2003 ASM International. All Rights Reserved. www.asminternational.org Practical Nitriding and Ferritic Nitrocarburizing (#06950G) CHAPTER 1 An Introduction to Nitriding THE NITRIDING PROCESS, first developed in the early 1900s, con- tinues to play an important role in many industrial applications. Along with the derivative nitrocarburizing process, nitriding often is used in the manufacture of aircraft, bearings, automotive components, textile machin- ery, and turbine generation systems. Though wrapped in a bit of “alchemi- cal mystery,” it remains the simplest of the case hardening techniques. The secret of the nitriding process is that it does not require a phase change from ferrite to austenite, nor does it require a further change from austenite to martensite. In other words, the steel remains in the ferrite phase (or cementite, depending on alloy composition) during the complete proce- dure. This means that the molecular structure of the ferrite (body-centered cubic, or bcc, lattice) does not change its configuration or grow into the face-centered cubic (fcc) lattice characteristic of austenite, as occurs in more conventional methods such as carburizing. Furthermore, because only free cooling takes place, rather than rapid cooling or quenching, no subsequent transformation from austenite to martensite occurs. Again, there is no molecular size change and, more importantly, no dimensional change, only slight growth due to the volumetric change of the steel sur- face caused by the nitrogen diffusion. What can (and does) produce distor- tion are the induced surface stresses being released by the heat of the process, causing movement in the form of twisting and bending. -

20. Iron and Steel Part II Copy
USES FOR TYPES OF STEELS • Low carbon steel (0.08 - 0.35 % carbon) is ductile with low brittleness. It is is used for ANCIENT IRON auto body parts, home appliances, tin cans, I beams for construction. AND STEEL • Medium carbon steel (0.35 - 0.5 % carbon) (Part II) is used as crankshaft, gears, railroad axels. (Part II) They are difficult to weld. • High carbon steel (> 0.5 % carbon) is used for railroad wheels and rails, wrenches, steel cable, tools, dies, piano wire etc. BLAST FURNACE CAST IRON (PIG IRON) • Contains 1.5 - 5 % carbon. • Its melting point is 1130 oC. • The metal will shatter with a hard blow. • Carbon in the form of graphite exists as flakes • Graphite serves as a lubricant (excellent bearing material). 1 Cast Grey Iron Blast furnace at Cast Grey Iron Karabük Iron works Cast Gray Iron Graphite flakes THE FINERY CAST IRON OBJECTS THE FINERY Cast cannon 15th. Cent. 2 INDIRECT METHOD OF STEEL BESSEMER PROCESS PRODUCTION (THE BESSEMER PROCESS) Crude cast iron from the blast furnace is remelted in a hearth (the finary) and subjected to a forced draught of air. The excess carbon is oxidized to carbon dioxide. The refined pig iron which is called malleable iron is now ready for final forging. BESSEMER PROCESS FORMS OF IRON CAST IRON Decarburization From Blast Furnace M.P. 1130oC 1,5-4.5 % C Bessemer STEEL Process M.P. 1400o C 0.1-0.9 % C WROUGHT IRON From Bloomery Cementation M.P 1535o C 0.06 % C Carburization 3 DIFFERENCES BETWEEN BLOOMERY ALLOY STEELS AND BLAST FURNACE • Increase in temperature (1300 - 1400oC) • Alloy steel describes steel that contain one or more alloying elements in addition to carbon. -

AP-42 12.13 Final Background Document for Steel Foundries
BACKGROUND REPORT AP-42 SECTION 12.13 STEEL FOUNDRIES Prepared for U.S. Environmental Protection Agency OAQPS/TSD/EIB Research Triangle Park, NC 27711 1-103 Pacific Environmental Services, Inc. P.O. Box 12077 Research Triangle Park, NC 27709 919/941-0333 1-103 AP-42 Background Report TECHNICAL SUPPORT DIVISION U.S. ENVIRONMENTAL PROTECTION AGENCY Office of Air Quality Planning and Standards Research Triangle Park, NC 27711 ii This report has been reviewed by the Technical Support Division of the Office of Air Quality Planning and Standards, EPA. Mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. Copies of this report are available through the Library Services Office (MD-35), U.S. Environmental Protection Agency, Research Triangle Park, NC 27711. iii TABLE OF CONTENTS 1.0 INTRODUCTION ................................................. 1 2.0 INDUSTRY DESCRIPTION ......................................... 2 2.1 GENERAL ................................................... 2 2.2 PROCESS DESCRIPTION1 ..................................... 2 2.3 EMISSIONS AND CONTROLS1,19 ................................ 8 2.4 REVIEW OF REFERENCES ..................................... 9 2.5 REFERENCES FOR CHAPTER 2 ............................... 11 3.0 GENERAL EMISSION DATA REVIEW AND ANALYSIS PROCEDURES ... 13 3.1 LITERATURE SEARCH AND SCREENING ....................... 13 3.2 EMISSION DATA QUALITY RATING SYSTEM ................... 14 3.3 EMISSION FACTOR QUALITY RATING SYSTEM ................. 16 3.4 -

AISI | Electric Arc Furnace Steelmaking
http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Home Steelworks Home Electric Arc Furnace Steelmaking By Jeremy A. T. Jones, Nupro Corporation SIGN UP to receive AISI's FREE e-news! Read the latest. Email: Name: Join Courtesy of Mannesmann Demag Corp. FURNACE OPERATIONS The electric arc furnace operates as a batch melting process producing batches of molten steel known "heats". The electric arc furnace operating cycle is called the tap-to-tap cycle and is made up of the following operations: Furnace charging Melting Refining De-slagging Tapping Furnace turn-around Modern operations aim for a tap-to-tap time of less than 60 minutes. Some twin shell furnace operations are achieving tap-to-tap times of 35 to 40 minutes. 10/3/2008 9:36 AM http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Furnace Charging The first step in the production of any heat is to select the grade of steel to be made. Usually a schedule is developed prior to each production shift. Thus the melter will know in advance the schedule for his shift. The scrap yard operator will prepare buckets of scrap according to the needs of the melter. Preparation of the charge bucket is an important operation, not only to ensure proper melt-in chemistry but also to ensure good melting conditions. The scrap must be layered in the bucket according to size and density to promote the rapid formation of a liquid pool of steel in the hearth while providing protection for the sidewalls and roof from electric arc radiation. -

CONTROL of DECARBURIZATION of STEEL Paul Shefsiek
CONTROL OF DECARBURIZATION OF STEEL Paul Shefsiek Introduction Historically, heating steel for forming, forging or rolling, was done in electrical resistance or natural gas heated furnaces. It was inevitable that these furnaces contained Oxygen and Decarburization of the steel surface occurred. This decarburization was either ignored or minimized by coating the steel with “ stopoff “. Also, this decarburization was minimized through the use nitrogen atmosphere furnaces. But, decarburization could not be reduced to acceptable limits until the Chemical Potential of the Carbon in the furnace atmosphere matched the dissolved Carbon in the steel. The Furnace Industry that provides Equipment for the processing of steel, because of the temperature ranges involved, divided itself into two categories, namely, Reheat and Heat Treating. Each has its own special Technology. But, because of this division, quite often one division does not know the Technology of the other. In some cases this has been unfortunate. If the Reheat side of the Industry had the Carburizing Technology of the Heat Treating side, this Technology could have been applied to applications where preventing Decarburizing was a requirement. Therefore, the purpose of the following discussion is to separate out that part of Carburizing Technology that is applicable to preventing Decarburization in Reheat applications. Fundamentals Control of the Decarburization of Steel has been standardized to the monitoring of - The Metal Temperature (Furnace Temperature) - The Atmosphere Concentration of Carbon Monoxide (CO) - The Atmosphere Concentration of Carbon Dioxide (CO2). Knowing the values of these three (3) parameters and the knowledge of - Saturated Austenite in Iron - The Alloy Content of the Steel - The Equilibrium Constant for the Controllable Chemical Reaction between the Steel and the Gas Atmosphere it can be determined if the Atmosphere will prevent Decarburization of the Steel. -

Induction Heating Applications the Processes, the Equipment, the Benefits CONTENTS
Induction heating applications The processes, the equipment, the benefits CONTENTS Introduction ..........................................................................3 Forging .................................................................................14 Induction coils ................................................................ 4-5 Melting .................................................................................15 Hardening ..............................................................................6 Straightening .....................................................................16 Tempering ..............................................................................7 Specialist applications ..................................................17 Brazing ....................................................................................8 How induction works .................................................................... 19 Bonding ..................................................................................9 Selecting the best solution ......................................... 20-21 Welding ................................................................................10 International certifications .................................................... 22 Annealing / normalizing ................................................................11 Some of our customers..................................................................23 Pre-heating ........................................................................12 -

1.1%Mn--0.05%C Steel Sheets by Silicon Dioxide and Development Of
Materials Transactions, Vol. 44, No. 6 (2003) pp. 1096 to 1105 #2003 The Japan Institute of Metals Decarburization of 3%Si–1.1%Mn–0.05%C Steel Sheets by Silicon Dioxide and Development of {100}h012i Texture* Toshiro Tomida Corporate Research & Development Laboratories, Sumitomo Metal Industries, Ltd., Amagasaki 660-0891, Japan Decarburization of silicon steel sheets by annealing with oxide separators has been found to cause a high degree of {100}-texture development. Cold-rolled Fe–3%Si–1.1%Mn–0.05%C sheets of 0.35 mm thickness were laminated with separators containing SiO2. They were then annealed under a reduced pressure at about 1300 K in a ferrite and austenite two phase region. It has been observed that carbon concentration notably decreases down to 0.001% during the lamination annealing. Thus an almost complete decarburization of sheet steels was possible, whereas no oxidation of silicon as well as manganese and iron occurred. Associated with decarburization, columnar ferrite grains grew inward from sheet surfaces due to the phase transformation from austenite to ferrite. A {100}h012i texture dramatically developed in the columnar grains. Fully decarburized materials consisted of grains of 0.6 mm diameter, more than 90% of which were closely aligned with {100}h012i orientation. Another aspect of great interest in the grain structure after decarburization was that there existed convoluted domains of a few mm in width, in which dozens of grains were oriented in a single variant of the texture, (100)[012] or (100)[021]. The decarburization is considered to be caused by the thermo-chemical reaction, 2C+SiO2!Si+2CO.