Minelli2020-Arthropod Segments and Segmentation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Supra-Familial Taxon Names of the Diplopoda Table 4A
Milli-PEET, Taxonomy Table 4 Page - 1 - Table 4: Supra-familial taxon names of the Diplopoda Table 4a: List of current supra-familial taxon names in alphabetical order, with their old invalid counterpart and included orders. [Brackets] indicate that the taxon group circumscribed by the old taxon group name is not recognized in Shelley's 2003 classification. Current Name Old Taxon Name Order Brannerioidea in part Trachyzona Verhoeff, 1913 Chordeumatida Callipodida Lysiopetalida Chamberlin, 1943 Callipodida [Cambaloidea+Spirobolida+ Chorizognatha Verhoeff, 1910 Cambaloidea+Spirobolida+ Spirostreptida] Spirostreptida Chelodesmidea Leptodesmidi Brölemann, 1916 Polydesmida Chelodesmidea Sphaeriodesmidea Jeekel, 1971 Polydesmida Chordeumatida Ascospermophora Verhoeff, 1900 Chordeumatida Chordeumatida Craspedosomatida Jeekel, 1971 Chordeumatida Chordeumatidea Craspedsomatoidea Cook, 1895 Chordeumatida Chordeumatoidea Megasacophora Verhoeff, 1929 Chordeumatida Craspedosomatoidea Cheiritophora Verhoeff, 1929 Chordeumatida Diplomaragnoidea Ancestreumatoidea Golovatch, 1977 Chordeumatida Glomerida Plesiocerata Verhoeff, 1910 Glomerida Hasseoidea Orobainosomidi Brolemann, 1935 Chordeumatida Hasseoidea Protopoda Verhoeff, 1929 Chordeumatida Helminthomorpha Proterandria Verhoeff, 1894 all helminthomorph orders Heterochordeumatoidea Oedomopoda Verhoeff, 1929 Chordeumatida Julida Symphyognatha Verhoeff, 1910 Julida Julida Zygocheta Cook, 1895 Julida [Julida+Spirostreptida] Diplocheta Cook, 1895 Julida+Spirostreptida [Julida in part[ Arthrophora Verhoeff, -

Biochemical Divergence Between Cavernicolous and Marine
The position of crustaceans within Arthropoda - Evidence from nine molecular loci and morphology GONZALO GIRIBET', STEFAN RICHTER2, GREGORY D. EDGECOMBE3 & WARD C. WHEELER4 Department of Organismic and Evolutionary- Biology, Museum of Comparative Zoology; Harvard University, Cambridge, Massachusetts, U.S.A. ' Friedrich-Schiller-UniversitdtJena, Instituifiir Spezielte Zoologie und Evolutionsbiologie, Jena, Germany 3Australian Museum, Sydney, NSW, Australia Division of Invertebrate Zoology, American Museum of Natural History, New York, U.S.A. ABSTRACT The monophyly of Crustacea, relationships of crustaceans to other arthropods, and internal phylogeny of Crustacea are appraised via parsimony analysis in a total evidence frame work. Data include sequences from three nuclear ribosomal genes, four nuclear coding genes, and two mitochondrial genes, together with 352 characters from external morphol ogy, internal anatomy, development, and mitochondrial gene order. Subjecting the com bined data set to 20 different parameter sets for variable gap and transversion costs, crusta ceans group with hexapods in Tetraconata across nearly all explored parameter space, and are members of a monophyletic Mandibulata across much of the parameter space. Crustacea is non-monophyletic at low indel costs, but monophyly is favored at higher indel costs, at which morphology exerts a greater influence. The most stable higher-level crusta cean groupings are Malacostraca, Branchiopoda, Branchiura + Pentastomida, and an ostracod-cirripede group. For combined data, the Thoracopoda and Maxillopoda concepts are unsupported, and Entomostraca is only retrieved under parameter sets of low congruence. Most of the current disagreement over deep divisions in Arthropoda (e.g., Mandibulata versus Paradoxopoda or Cormogonida versus Chelicerata) can be viewed as uncertainty regarding the position of the root in the arthropod cladogram rather than as fundamental topological disagreement as supported in earlier studies (e.g., Schizoramia versus Mandibulata or Atelocerata versus Tetraconata). -

A New Genus of the Millipede Tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean Region
European Journal of Taxonomy 70: 1-12 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2013.70 www.europeanjournaloftaxonomy.eu 2013 · Lazányi E. & Vagalinski B. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:5E23F454-2A68-42F6-86FB-7D9952B2FE7B A new genus of the millipede tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean region Eszter LAZÁNYI1,4 & Boyan VAGALINSKI2,3,5 1 Corresponding author: Department of Zoology, Hungarian Natural History Museum, Baross u. 13, H-1088 Budapest, Hungary. E-mail: [email protected] 2 Faculty of Biology, Sofia University, 8 Dragan Tsankov Blvd., 1164 Sofia, Bulgaria. E-mail: [email protected] 3 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Yurii Gagarin Street, 1113, Sofia, Bulgaria. 4 urn:lsid:zoobank.org:author:02DB48F1-624C-4435-AF85-FA87168CD85A 5 urn:lsid:zoobank.org:author:973B8725-039E-4F29-8D73-96A7F52CF934 Abstract. A new genus of the julid tribe Brachyiulini, Enghophyllum gen. nov., is described, comprising two species from Greece. The type-species, E. naxium (Verhoeff, 1901) comb. nov. (ex Megaphyllum Verhoeff, 1894), appears to be rather widespread in the Aegean: it is known from Antiparos Island and Naxos Island (the type locality), both in the Cyclades, as well as East Mavri Islet, Dodecanese Archipelago (new record). The vulva of E. naxium is described for the first time. In addition,E. sifnium gen. et sp. nov. is described based on a single adult male from Sifnos Island, Cyclades. The new genus is distinct from other genera of the Brachyiulini mainly by its peculiar gonopod structure, apparently disjunct and at least mostly apomorphous: (1) promeres broad, shield-like, in situ protruding mostly posteriad, completely covering the opisthomeres and gonopodal sinus; (2) transverse muscles and coxal apodemes of promere fully reduced; (3) opisthomere with three differentiated processes, i.e., lateral, basal posterior and apical posterior; (4) solenomere rather simple, tubular. -

Centre International De Myriapodologie
N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Mus6umNationald'HistoireNaturelle,Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-75231 ParisCedex05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISIES REPE&TORIEES PANS LA BASE PASCAL DE L' INIST 1995 N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Museum National d'Histoire N aturelle, Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-7 5231 Paris Cedex 05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISTES REPERTORIEES DANS LA BASE PASCAL DE L' INIST 1995 SOMMAIRE CONTENTS ZUSAMMENFASSUNG Pages Seite lOth INTERNATIONAL CONGRESS OF MYRIAPODOLOGY .................................. 1 9th CONGRES INTERNATIONAL DE MYRIAPODOLOGIE.................................................... 1 Contacter le Secretariat permanent par E-M AIL & FA X............................................................ 1 The Proceedings of the 9th International Congress of Myriapodology...................... 2 MILLEPATTIA, sommaire .du prochain bulletin....................................................................... 2 Obituary: Colin Peter FAIRHURST (1942-1994) ............................................................. 3 BULLETIN of the -

Cell Size Versus Body Size in Geophilomorph Centipedes
Sci Nat (2015) 102:16 DOI 10.1007/s00114-015-1269-4 ORIGINAL PAPER Cell size versus body size in geophilomorph centipedes Marco Moretto1 & Alessandro Minelli1 & Giuseppe Fusco1 Received: 22 December 2014 /Revised: 9 March 2015 /Accepted: 12 March 2015 # Springer-Verlag Berlin Heidelberg 2015 Abstract Variation in animal body size is the result of a com- Introduction plex interplay between variation in cell number and cell size, but the latter has seldom been considered in wide-ranging Total body size and the relative dimensions of body parts are comparative studies, although distinct patterns of variation key attributes that shape most of a species’ functions, behav- have been described in the evolution of different lineages. iour, and ecological relationships. However, the developmen- We investigated the correlation between epidermal cell size tal mechanisms that control size and shape are still unexplored and body size in a sample of 29 geophilomorph centipede or incompletely understood in most animal taxa (Nijhout and species, representative of a wide range of body sizes, from Callier 2015). 6 mm dwarf species to gigantic species more than 200 mm In the few animal species in which adequate studies have long, exploiting the marks of epidermal cells on the overlying been performed, intraspecific variation in body size is the cuticle in the form of micro-sculptures called scutes. We found result of a complex interplay between variation in cell number conspicuous and significant variation in average scute area, and variation in cell size (e.g. Robertson 1959; Partridge et al. both between suprageneric taxa and between genera, while 1994;DeMoedetal.1997; Azevedo et al. -
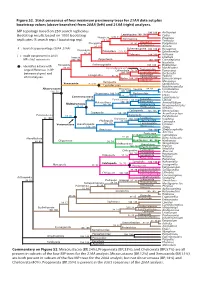
Figure S2. Strict Consensus of Four Maximum Parsimony Trees for 21AA Data Set Plus Bootstrap Values (Above Branches) from 20AA (Left) and 21AA (Right) Analyses
Figure S2. Strict consensus of four maximum parsimony trees for 21AA data set plus bootstrap values (above branches) from 20AA (left) and 21AA (right) analyses. MP topology based on 250 search replicates. 100 100 Antheraea Bootstrap results based on 1000 bootstrap Lepidoptera 100 100 Cydia Neoptera [23] 44 Dermaptera Prodoxus replicates (5 search reps / bootstrap rep). 68 66 Forficula Blattodea Pterygota 92 96 Periplaneta Orthoptera 96 97 Acheta # : bootstrap percentage (20AA 21AA) Ephemeroptera Dicondylia 100 100 Hexagenia Paleoptera [37] 52 Ephemerella 98 98 Odonata 100 100 Ischnura [ ] : node not present in 20AA Insecta Libellula MP strict consensus 100 100 Zygentoma 100 100 Ctenolepisma Nicoletia Archaeognatha : Identifies 6 taxa with Hexapoda 100 100 Pedetontus 89 86 Machiloides Entomobryomorpha Tomoceridae large differences in BP Collembola 70 76 Tomocerus between degen1 and 100 100 Entomobryidae Orchesella Entognatha 82 82 Poduridae Podura 20AA analyses. Diplura 100 100 Campodeidae Japygidae Eumesocampa Remipedia Metajapyx Xenocarida [31] 51 Speleonectes Cephalocarida Hutchinsoniella Altocrustacea Thoracica Sessilia 88 83 Semibalanus [14] 31 100 100 Chthamalus Thecostraca 100 100 Pedunculata Lepas Communostraca Rhizocephala Loxothylacus 96 89 Eumalacostraca 84 85 Eucarida Libinia Malacostraca 100 100 Peracarida Armadillidium Multicrustacea 100 100 Hoplocarida 69 84 Neogonodactylus Phyllocarida Nebalia Cyclopoida 100 100 Mesocyclops Copepoda 100 100 Acanthocyclops Pancrustacea 65 82 Calanoida Eurytemora 96 100 Diplostraca 100 100 -

Exploring Phylogenomic Relationships Within Myriapoda: Should High Matrix Occupancy Be the Goal?
bioRxiv preprint doi: https://doi.org/10.1101/030973; this version posted November 9, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Exploring phylogenomic relationships within Myriapoda: should high matrix occupancy be the goal? ROSA FERNÁNDEZ1, GREGORY D. EDGECOMBE2 AND GONZALO GIRIBET1 1Museum of Comparative Zoology & Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA 2Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK 1 bioRxiv preprint doi: https://doi.org/10.1101/030973; this version posted November 9, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract.—Myriapods are one of the dominant terrestrial arthropod groups including the diverse and familiar centipedes and millipedes. Although molecular evidence has shown that Myriapoda is monophyletic, its internal phylogeny remains contentious and understudied, especially when compared to those of Chelicerata and Hexapoda. Until now, efforts have focused on taxon sampling (e.g., by including a handful of genes in many species) or on maximizing matrix occupancy (e.g., by including hundreds or thousands of genes in just a few species), but a phylogeny maximizing sampling at both levels remains elusive. In this study, we analyzed forty Illumina transcriptomes representing three myriapod classes (Diplopoda, Chilopoda and Symphyla); twenty-five transcriptomes were newly sequenced to maximize representation at the ordinal level in Diplopoda and at the family level in Chilopoda. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Chilopoda) from Central and South America Including Mexico
AMAZONIANA XVI (1/2): 59- 185 Kiel, Dezember 2000 A catalogue of the geophilomorph centipedes (Chilopoda) from Central and South America including Mexico by D. Foddai, L.A. Pereira & A. Minelli Dr. Donatella Foddai and Prof. Dr. Alessandro Minelli, Dipartimento di Biologia, Universita degli Studi di Padova, Via Ugo Bassi 588, I 35131 Padova, Italy. Dr. Luis Alberto Pereira, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s.n., 1900 La Plata, R. Argentina. (Accepted for publication: July. 2000). Abstract This paper is an annotated catalogue of the gcophilomorph centipedes known from Mexico, Central America, West Indies, South America and the adjacent islands. 310 species and 4 subspecies in 91 genera in II fam ilies are listed, not including 6 additional taxa of uncertain generic identity and 4 undescribed species provisionally listed as 'n.sp.' under their respective genera. Sixteen new combinations are proposed: GaJTina pujola (CHAMBERLIN, 1943) and G. vera (CHAM BERLIN, 1943), both from Pycnona; Nesidiphilus plusioporus (ATT EMS, 1947). from Mesogeophilus VERHOEFF, 190 I; Po/ycricus bredini (CRABILL, 1960), P. cordobanensis (VERHOEFF. 1934), P. haitiensis (CHAMBERLIN, 1915) and P. nesiotes (CHAMBERLIN. 1915), all fr om Lestophilus; Tuoba baeckstroemi (VERHOEFF, 1924), from Geophilus (Nesogeophilus); T. culebrae (SILVESTRI. 1908), from Geophilus; T. latico/lis (ATTEMS, 1903), from Geophilus (Nesogeophilus); Titanophilus hasei (VERHOEFF, 1938), from Notiphilides (Venezuelides); T. incus (CHAMBERLIN, 1941), from lncorya; Schendylops nealotus (CHAMBERLIN. 1950), from Nesondyla nealota; Diplethmus porosus (ATTEMS, 1947). from Cyclorya porosa; Chomatohius craterus (CHAMBERLIN, 1944) and Ch. orizabae (CHAMBERLIN, 1944), both from Gosiphilus. The new replacement name Schizonampa Iibera is proposed pro Schizonampa prognatha (CRABILL. -

A New Species of Illacme Cook & Loomis, 1928
A peer-reviewed open-access journal ZooKeys 626: 1–43A new (2016) species of Illacme Cook and Loomis, 1928 from Sequoia National Park... 1 doi: 10.3897/zookeys.626.9681 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida) Paul E. Marek1, Jean K. Krejca2, William A. Shear3 1 Virginia Polytechnic Institute and State University, Department of Entomology, Price Hall, Blacksburg, Virginia, USA 2 Zara Environmental LLC, 1707 W FM 1626, Manchaca, Texas, USA 3 Hampden-Sydney College, Department of Biology, Gilmer Hall, Hampden-Sydney, Virginia, USA Corresponding author: Paul E. Marek ([email protected]) Academic editor: R. Mesibov | Received 25 July 2016 | Accepted 19 September 2016 | Published 20 October 2016 http://zoobank.org/36E16503-BC2B-4D92-982E-FC2088094C93 Citation: Marek PE, Krejca JK, Shear WA (2016) A new species of Illacme Cook & Loomis, 1928 from Sequoia National Park, California, with a world catalog of the Siphonorhinidae (Diplopoda, Siphonophorida). ZooKeys 626: 1–43. doi: 10.3897/zookeys.626.9681 Abstract Members of the family Siphonorhinidae Cook, 1895 are thread-like eyeless millipedes that possess an astounding number of legs, including one individual with 750. Due to their cryptic lifestyle, rarity in natural history collections, and sporadic study over the last century, the family has an unclear phylogenetic placement, and intrafamilial relationships remain unknown. Here we report the discovery of a second spe- cies of Illacme, a millipede genus notable for possessing the greatest number of legs of any known animal on the planet. -

Accepted Manuscript Macroecological Inferences on Soil Fauna Through Comparative Niche Modeling: the Case of Hormogastridae (Annelida, Oligochaeta) Daniel F
This is an Open Access document downloaded from ORCA, Cardiff University's institutional repository: http://orca.cf.ac.uk/91465/ This is the author’s version of a work that was submitted to / accepted for publication. Citation for final published version: Marchán, Daniel F., Refoyo, Pablo, Fernández, Rosa, Novo Rodriguez, Marta, de Sosa, Irene and Díaz Cosín, Darío J. 2016. Macroecological inferences on soil fauna through comparative niche modeling: The case of Hormogastridae (Annelida, Oligochaeta). European Journal of Soil Biology 75 , pp. 115-122. 10.1016/j.ejsobi.2016.05.003 file Publishers page: http://dx.doi.org/10.1016/j.ejsobi.2016.05.003 <http://dx.doi.org/10.1016/j.ejsobi.2016.05.003> Please note: Changes made as a result of publishing processes such as copy-editing, formatting and page numbers may not be reflected in this version. For the definitive version of this publication, please refer to the published source. You are advised to consult the publisher’s version if you wish to cite this paper. This version is being made available in accordance with publisher policies. See http://orca.cf.ac.uk/policies.html for usage policies. Copyright and moral rights for publications made available in ORCA are retained by the copyright holders. Accepted manuscript Macroecological inferences on soil fauna through comparative niche modeling: the case of Hormogastridae (Annelida, Oligochaeta) Daniel F. Marchán1*#, Pablo Refoyo1#, Rosa Fernández2, Marta Novo3, Irene de Sosa1, Darío J. Díaz Cosín1 DOI: 10.1016/j.ejsobi.2016.05.003 To appear in: European Journal of Soil Biology Received date: 25 January 2016 Revised date: 12 May 2016 Accepted date: 17 May 2016 Please cite this article as: Marchán DF, Refoyo P, Fernández R, Novo M, de Sosa I, Díaz Cosín DJ (2016). -

Ordinal-Level Phylogenomics of the Arthropod Class
Ordinal-Level Phylogenomics of the Arthropod Class Diplopoda (Millipedes) Based on an Analysis of 221 Nuclear Protein-Coding Loci Generated Using Next- Generation Sequence Analyses Michael S. Brewer1,2*, Jason E. Bond3 1 Department of Environmental Science, Policy, and Management, University of California Berkeley, Berkeley, California, United States of America, 2 Department of Biology, East Carolina University, Greenville, North Carolina, United States of America, 3 Department of Biological Sciences and Auburn University Museum of Natural History, Auburn University, Auburn, Alabama, United States of America Abstract Background: The ancient and diverse, yet understudied arthropod class Diplopoda, the millipedes, has a muddled taxonomic history. Despite having a cosmopolitan distribution and a number of unique and interesting characteristics, the group has received relatively little attention; interest in millipede systematics is low compared to taxa of comparable diversity. The existing classification of the group comprises 16 orders. Past attempts to reconstruct millipede phylogenies have suffered from a paucity of characters and included too few taxa to confidently resolve relationships and make formal nomenclatural changes. Herein, we reconstruct an ordinal-level phylogeny for the class Diplopoda using the largest character set ever assembled for the group. Methods: Transcriptomic sequences were obtained from exemplar taxa representing much of the diversity of millipede orders using second-generation (i.e., next-generation or high-throughput) sequencing. These data were subject to rigorous orthology selection and phylogenetic dataset optimization and then used to reconstruct phylogenies employing Bayesian inference and maximum likelihood optimality criteria. Ancestral reconstructions of sperm transfer appendage development (gonopods), presence of lateral defense secretion pores (ozopores), and presence of spinnerets were considered.