Influence of Nutrient Loading on the Invasion of an Alien
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hamilton County (Ohio) Naturalization Records – Surname M
Hamilton County Naturalization Records – Surname M Applicant Age Country of Origin Departure Date Departure Port Arrive Date Entry Port Declaration Dec Date Vol Page Folder Naturalization Naturalization Date Maag, Frederick 46 Oldenburg Bremen New Orleans T 11/01/1852 5 132 F F Maag, Frederick 46 Oldenburg Bremen New Orleans T 11/01/1854 6 258 F F Maag, Sebastian 31 Baden Liverpool New York T 01/02/1858 16 296 F F Maahan, James 32 Ireland Toronto Buffalo T 01/20/1852 24 37 F F Maas, Anton 30 Prussia Bremen New York T 01/17/1853 7 34 F F Maas, Carl 18 Mannheim, Germany ? ? T 05/04/1885 T F Maas, Garrett William 25 Holland Rotterdam New York T 11/15/1852 5 287 F F Maas, Garrett William 25 Holland Rotterdam New York T 11/15/1852 6 411 F F Maas, Jacob 55 Germany Rotterdam New York F ? T T Maas, John 55 Germany Havre New York F ? T T Maas, John William 28 Holland Rotterdam New Orleans T 09/27/1848 22 56 F F Maas, Julius J. 48 Germany Bremen New York F ? T T Maas, Leonardus Aloysius 37 Holland Rotterdam New Orleans T 03/01/1852 24 6 F F Maass, F.W. 45 Hanover Bremen Baltimmore T 11/02/1860 18 3 F F Macalusa, Michael 23 Italy Palermo New York T 3/18/1903 T F MacAvoy, Henry 48 England Liverpool New York T 10/26/1891 T F MacDermott, Joseph England ? ? T 2/26/1900 T F Machenheimer, Christoph 29 Hesse Darmstadt Havre New York T 02/19/1850 2 100 F F Machnovitz, Moses 50 Russia Bremen Baltimore T 4/27/1900 T F Maciejensky, Martin 28 Prussia Hamburg New York T 04/28/1854 8 280 F F Maciejensky, Martin 28 Prussia Hamburg New York T 04/28/1854 9 151 F -
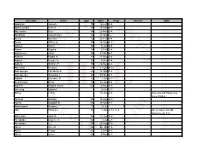
Surname Given Age Date Page Maiden Note Abdullah Joseph 70 5-Feb A-8 Abercrombie Bert H
Surname Given Age Date Page Maiden Note Abdullah Joseph 70 5-Feb A-8 Abercrombie Bert H. 88 29-Dec B-9 Abernathy Kate 84 22-Nov B-4 Abraham Joseph Ben 86 21-Mar D-2 Acela Michael 73 23-Feb A-4 Achor Arthur A. 58 16-Sep A-11 Adalay Steve 92 6-Jan B-6 Adam Sophia 78 3-Feb B-4 Adamczyk John 85 21-Oct B-7 Adams Edwin B. 81 27-Aug B-4 Adank Cassie R. 75 7-Dec A-4 Adank William F. 76 24-Dec A-8 Adelman Irving D. 59 31-Oct D-10 Adelsperger Elizabeth A. 60 18-Mar A-8 Adelsperger Susanna T. 82 25-Oct A-7 Adoba Michael, Sr. 80 1-Jun A-12 Aeschliman Betty 32 18-Jan B-4 Aguirre Regina Avilla 63 4-Nov A-6 Ahlering Edward 6-Oct C-7 Ahley Lillian 15-Jun A-6 Also spelled Haley see June 16 E-2 Ahmed Hassan 68 13-Aug A-9 Akers Edward W. 66 16-Mar A-7 Aksentijevic Rodney 15 8-Jul 1 Alb Florence 70 1-Jun A-12, C-5 Gives name as Alb Florence on C-5 Albertson Jack R. 59 11-Jun C-2 Alexander Eugene A. 62 7-Jul C-8 Alexander L.C. 58 20-Aug B-3 Allen Cleo D. 66 26-Jan A-6 Allen Frosty 65 2-Dec B-6 Allen Grace 65 9-Nov B-3 Allen James Virgil 55 19-Aug A-4 Allen Weber 62 2-Mar A-4 Alley George Wesley 54 4-Jan A-5 Alonzo Maria 73 15-Oct B-5 Altshuller Nathan D. -

Tackling Racism Seriously
Institut de Drets Humans / Facultat de Dret Doctor’s degree in Human rights, democracy and international justice TACKLING RACISM SERIOUSLY HUMAN RIGHTS AND THE UNDERPINNINGS OF ETHNIC RECOGNITION Doctoral thesis Author Supervisor Pier-Luc Dupont Ángeles Solanes Corella València, May 2017 Because they are rational creatures, sailors go to sea with the calculations already done; and all rational creatures go out on the sea of life with their minds made up on the common questions of right and wrong, as well as on many of the much harder questions of wise and foolish. And we can presume that they will continue to do so long as foresight continues to be a human quality. Whatever we adopt as the fundamental principle of morality, we need subordinate principles through which to apply it. John Stuart Mill It is important to be quite careful before seeing any tension between equality and liberty. Tension exists only when we specify conceptions of these broad terms that cannot peacefully coexist. Perhaps such incompatible conceptions cannot be defended. Perhaps the best conceptions of equality are entirely compatible with the best understandings of liberty. Cass Sunstein Neither in interpreting statutes nor precedents are judges confined to the alternatives of blind, arbitrary choice, or ‘mechanical’ deduction from rules with predetermined meaning. Very often their choice is guided by an assumption that the purpose of the rules which they are interpreting is a reasonable one, so that the rules are not intended to work injustice or offend settled moral principles. HLA Hart Rights is the child of law; from real law come real right; but from imaginary laws, from ‘laws of nature’, come imaginary rights… Natural rights is simple nonsense […], nonsense upon stilts. -

Mathieu MEUNIER Evaluation Du Rôle De La Niche Hématopoïétique Dans L
THÈSE Pour obtenir le grade de DOCTEUR DE LA COMMUNAUTÉ UNIVERSITÉ GRENOBLE ALPES Spécialité : MBS - Modèles, méthodes et algorithmes en biologie, santé et environnement Arrêté ministériel : 25 mai 2016 Présentée par Mathieu MEUNIER Thèse dirigée par Sophie PARK, Communauté Université Grenoble Alpes préparée au sein du Laboratoire CRI IAB - Centre de Recherche Oncologie/Développement - Institute for Advanced Biosciences dans l'École Doctorale Ingénierie pour la santé la Cognition et l'Environnement Evaluation du rôle de la niche hématopoïétique dans l'induction des syndromes myélodysplasiques: rôle de dicer1 et du stress oxydatif The implication of hematopoietic niche in induction of myelodysplastic syndromes: the role of Dicer1 and oxidative stress Thèse soutenue publiquement le 5 avril 2018, devant le jury composé de : Madame SOPHIE PARK PROFESSEUR DES UNIV - PRATICIEN HOSP., CHU GRENOBLE ALPES, Directeur de thèse Madame CATHERINE LACOMBE PROFESSEUR EMERITE, ASSISTANCE PUBLIQUE - HOPITAUX DE PARIS, Examinateur Monsieur PIERRE HAINAUT PROFESSEUR, UNIVERSITE GRENOBLE ALPES, Examinateur Madame PATRICIA AGUILAR MARTINEZ PROFESSEUR DES UNIV - PRATICIEN HOSP., UNIVERSITE DE MONTPELLIER, Rapporteur Monsieur JEAN-FRANÇOIS PEYRON DIRECTEUR DE RECHERCHE, UNIVERSITE DE NICE, Rapporteur Monsieur JEAN-YVES CAHN PROFESSEUR DES UNIV - PRATICIEN HOSP., CHU GRENOBLE ALPES, Président REMERCIEMENTS Je remercie Madame Patricia Aguilar-Martinez, Madame Catherine Lacombe, Monsieur Jean- François Peyron et Monsieur Pierre Hainaut pour avoir accepté d’être membre du jury. Merci pour le temps que vous avez consacré à lire et évaluer mon travail de thèse. Ce travail n’aurait pas été possible sans le soutien de l’Université Grenoble Alpes et l’association ARAMIS Alpes qui m’ont permis grâce à une allocation d’une bourse de me consacrer sereinement et entièrement à l’élaboration de ma thèse. -

E-Poster List
E-POSTER LIST The complete list of abstracts accepted as e-posters for the 29th EADV Congress, 29th – 31st October, 2020 Acne and Related Disorders, Hidradenitis Suppurativa P0001 Lupus Milliaris Disseminatus Faciei : A rare case treated with Isotretinoin Jesmin A. (DHAKA, Bangladesh) P0002 A comparative study of microneedling alone versus along with platelet rich plasma in acne scars Debbarman K., Gupta M. (Delhi, India) P0003 Serum levels of 25-hydroxyvitamin D and IL17 and their association with acne severity in patients with severe and very severe acne vulgaris Elkamshoushi A., Elneily D., Ismail S., Mahmoud H. (Alexandria, Egypt) P0006 The psychosocial impact of Hidradenitis suppurativa Vasileva S., Vasilev S., Vasilev B., Brishkoska Boshkovski V., Vasileva M. (Shtip, Macedonia) P0008 Myths, Perceptions and Practices in Acne: A Study on Adolescents and Young Adults Yorulmaz A., Yalçın B. (Ankara, Turkey) P0009 Adalimumab in severe hidradenitis suppurativa - A case report of a novel experiencr Balendran T., Gunasekera C. (Batticaloa, Sri Lanka) P0010 Relationship between acne vulgaris and insulin resistance markers and effect of isotretinoin on insulin resistance markers in patients with acne vulgaris: A systematic review and meta- analysis Raizada A. (Bhubaneswar , India) P0011 Systemic steroids in moderate-to-severe hidradenitis suppurativa Duarte B., Cunha N., Lencastre A., Cabete J. (Lisbon, Portugal) P0012 Ixekizumab restores clinical response in patients with secondary loss of efficacy from Adalimumab: results of a case series of 10 patients with Hidradenitis suppurativa Hilbring C., Augustin M., Girbig G., Kirsten N. (Hamburg, Germany) P0013 Microbiological assessment of macrolides and lincosamides effectiveness in the treatment of folliculitis and acne in Ukraine Voloshynovych M., Kutsyk R., Chmut V., Yurchyshyn O., Pavliuk N., Rusko H. -

Detection and Isolation of Plasmodium Liver Stages and Analysis of Circumsporozoite Protein Antigen Processing
STUDIES ON EXOERYTHROCYTIC DEVELOPMENT OF PLASMODIUM FALCIPARUM IN VITRO: DETECTION AND ISOLATION OF PLASMODIUM LIVER STAGES AND ANALYSIS OF CIRCUMSPOROZOITE PROTEIN ANTIGEN PROCESSING by Peter Dumoulin A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland May, 2015 Abstract Malaria, the disease caused by Plasmodium parasites, remains a major global health burden despite efforts to eradicate the parasite. Infection is initiated when a mosquito deposits sporozoites into the dermis that then actively invade hepatocytes. Sporozoites and the resulting liver stage of Plasmodium falciparum infection are leading targets for generation of a protective vaccine. The circumsporozoite protein is the major surface protein on sporozoites and is the target of vaccines aimed at preventing blood stage infection. During the process of invasion, sporozoites cleave the N-terminus of CSP and consequently two forms of CSP are potentially exposed to the immune system. We utilized ectopic expression of two natural form of CSP to quantify their abilities to serve as targets for MHC class I-restricted CD8+ T cells. We determined that presentation of both forms of CSP on MHC class I depends on proteasomal activity, however, presentation of full length CSP is more efficient and is conferred by the presence of N-terminal lysine residues absent in the cleaved form of CSP. To evaluate the presence of these two forms during in vitro infection, we developed methods to allow for detection and isolation of developing live P. falciparum liver stages by flow cytometry. Using this technique we compared the susceptibility of five immortalized human hepatocyte cell lines and primary hepatocyte cultures from three donors to infection by P. -

Annual Conference
67th Annual Conference Evaluating New Frontiers in Public Opinion and Social Research Conference Program May 17–20, 2012 JW Marriott Orlando Grande Lakes • Orlando, Florida www.aapor.org AAPOR 12 FP.indd 1 5/2/12 11:24 AM Thursday, May 17, 2012 1:30 p.m. - 3:00 p.m. Concurrent Sesson A New Frontiers: Interactive and Gaming Techniques to Improve Surveys Interactive and Gaming Techniques to Improve Surveys Elizabeth Dean, RTI International ([email protected]); Adam Sage, RTI International ([email protected]); Jennie W. Lai, Nielsen ([email protected]); Michael Link, Nielsen ([email protected]); Ashley Richards, RTI International ([email protected]); Lorelle Vanno, Nielsen ([email protected]); Jeffrey Henning, Affinova, Inc. ([email protected]) The mobile, digitally networked era has expanded communication tools and styles. Technological augmentation of social networks enables screening calls, blocking contacts, and avoiding face to face interaction. These technologies also facilitate active and passive sharing of massive amounts of personal information while building virtual communities to meet real social needs. Online communication styles signify the arrival of a model for surveying very different from the two-way “conversation with a purpose.” One norm of online communication is interactivity. Facebook’s model of status updates, user comments, “likes,” and live feeds of social conversation can now be considered a norm for online communication. A second norm of online communication is the game. People compete in online games to earn rewards, status, and achievement but also to express themselves, build community, and experience altruism. “Gamification” makes routine experiences more engaging by providing points, badges and status for behaviors like checking in to venues (Foursquare) and buying coffee (My Starbucks Rewards). -

2015 Annual Report
t r o p e R l a u n An 2015 2015 Board of Directors Officers Joel Huotari President Emily Hartzog 1st Vice President Kurt Broski 2nd Vice President Dayton B. Smith III Treasurer Colleen Anderson Secretary Sam Castree Past President Sarah Wolf Executive Director Directors Growing…Volunteers! Brian Blakemore Discovery Center has grown from our foundational roots which Nathan Boelkins were started by volunteers. Our community, whether small or Karen Brown Lesly Couper tall, are a vital resource for helping us advance our mission! Tony Foti James Horton total volunteers Ehren Jarrett 482 Scott Kaiser active Youth Experiencing Science (YES) Michelle Klaman volunteers (ages 13-17) Kelly Lattimer 150 Carol Mittel Tony Moczynski individual adults Lindsay Moore 40 Denise Noe Board of Director members contributed Lisa A. Normoyle over hours of their expertise James Pirages 24 670 Jeffery Weatherall business and school groups Gordon Wright 26 Auxiliary volunteer hours contributed to the museum Committee Members Deb Boland 5500+ Sarah Butenhoff Audra Capriola Christina Elbers Growing...Recognition! Nina Jacobson Discovery Center was awarded a TripAdvisor® Certificate Laura Kenrick of Excellence and was recognized as a Top Family Julia Kindler Attraction Worth Traveling For in the Andrea Mandala U.S. by Flipkey/TripAdvisor. Bill Miller Jennie Polizzotto Rockford Register Star annual What Rocks contest Catherine Povalitis Best Child-friendly Place Liz Wood B103 Radio Station Carol Wright Best Place to take the Kids in Rockford 2 DISCOVERY CENTER MUSEUM ANNUAL REPORT 2015 Growing…Exploration! Power of Electricity This new exhibit provides high quality 21st century learning experiences in STEAM, inspiring the next generation through interaction and energy stimulation. -

Saint Joseph County Cemetery Inscriptions
Saint Joseph County, Indiana Cemetery Inscriptions Surname, Given, Volume #, Page # M __?__,CHARLES E.,2,29 M __?__,IDA,2,29 M?,FRANKIE S.,3,277 M?,S.A.,3,293 MacDonald,Grace A.,5,87 MACDONALD,JACK G.,4,184 MACDONALD,MARY C.,4,184 MACELREE,HARRIET G.,3,201 MacGLENN,DONNIE,4,51 MACHEMER,JAMES W.,4,72 MACHEMER,MATILDA W.,4,72 MacJANNET,JULIA,4,115 MACK,ADA FLORENCE,3,99 MACK,CHARITY L.,1,17 MACK,FLORENCE,1,17 MACK,JOHN H.,1,17 MACK,JOHN W.,1,17 MACK,LEE,4,88 MACK,LEO W.,3,148 MACK,MAGGIE,3,99 MACK,PAULINE S.,3,148 MACK,REUBEN S.,1,17 MACK,SARAH L.,1,17 MACK,STELLA,4,88 MACK,THOMAS,3,99 MACK,THOMAS LEROY,3,99 MACK,WALTER J.,1,17 MACKEY,GEORGE M.,4,96 MACKEY,STELLA B.,4,96 MACKIN,ANNA M.,1,240 MACKIN,EALR,1,240 MACKIN,VADA,1,240 MACOMBER,ELIZABETH B.,1,203 MacQUIRE,CATHERINE A. SCHLARB,4,53 MACRELLI,JOHN,2,184 MACRELLI,JOSEPH R.,3,154 MACRELLI,LENA,2,184 MACRIGGS,LAVERNE,3,212 MACULSKI,CLARA,3,131 MACULSKI,EDWARD J.,3,132 MACULSKI,JOHN L.,2,180 MACULSKI,JOSEPH,3,131 MACUMBER,ANNA,2,77 MACUMBER,ESTHER,2,77 MACUMBER,LYMAN,2,77 MACUMBER,MARY M.,2,77 MACUMBER,SAMUEL S.,2,77 MACUSZONAK,LAWRENCE,3,145 MACUSZONOK,ANTONINA,3,146 MACUSZONOK,BLAZEJ,3,146 MADARAS,JOSEPH W.,3,116 MADARAS,MAMIE R.,3,116 MADDEN,CECIL DEWEY,4,147 MADDEN,FLORENCE LOUISE,4,147 MADDEN,ROBERT DEWEY,4,147 MADDON,EDWARD E.,3,208 (c) 1999-2007 South Bend Area Genealogical Society - All Rights Reserved May not be copied or reprinted without permission. -

PROGRAM BOOK LISBON 2018 Join Usinlisbon Congress on 11 Lisbon, Portugal, 16-20 May Portugal, Lisbon, 2018 Th International Autoimmunity.Kene S.Com PROGRAM BOOK
PROGRAM BOOK Join Us in Lisbon 11th International Congress on Lisbon, Portugal, 16-20 May 2018 LISBON 2018 LISBON autoimmunity.kenes.com PROGRAM BOOK CONGRESS ORGANIZERS Rue François-Versonnex 7, CH-1207 Geneva, SWITZERLAND Tel: +41 22 908 0488 Fax: +41 22 906 9140 Email: [email protected] Website: autoimmunity.kenes.com TABLE OF CONTENTS Welcome Message 3 Committees 4 Timetable 6 General Information 10 CME/CPD Accreditation 12 Information for Presenters 15 E-Poster Discussion Sessions 16 Venue Maps 18 About Leipzig 20 Awards 21 Networking Events 26 Congress App 27 4th International Symposium on Vaccines 28 Basic Immunology Course 29 Patient Forum 30 SCIENTIFIC PROGRAM & E-POSTER DISCUSSIONS Wednesday, April 6 33 Thursday, April 7 39 Friday, April 8 81 Saturday, April 9 129 Sunday, April 10 177 Index 187 ACKNOWLEDGEMENTS & INDUSTRY SUPPORT Acknowledgements 211 Industry Symposia 214 Exhibition Map 218 List of Exhibitors 219 Supporter & Exhibitor Profiles 223 2 AUTOIMMUNITY 2016 WELCOME MESSAGE Dear Friends, The International Congress on Autoimmunity has reached a historical moment: in April 2016 more than 2000 of the world's autoimmunologists have gathered for the 10th time to exchange knowledge about the more than 80 autoimmune diseases. The meeting point this time is the artistic city of Leipzig, Germany, known for its long tradition in trade fairs and its compelling selection of museums, musical events and other cultural offerings. Our loyal participants are already familiar with the high level of medical science that awaits them at the International Congresses on Autoimmunity and our newcomers will be impressed by the diversity of excellent sessions offered on a variety of topics, ranging from basic research to novel diagnostic and treatment methods of autoimmune diseases. -

Catalog 4Thiabr En.Pdf
Content P. 7 Preface P. 13 The Open City - Curatorial Statement P. 19 The Biennale and the City P. 25 Open City: Designing Coexistence P. 27 The Forum P. 35 Maakbaarheid ('ma:kba:rɦɛ:it) P. 39 Refuge P. 45 Reciprocity P. 49 Community P. 53 Squat P. 59 Collective P. 63 Open City Event Program P. 65 Open City: Designing Coexistence – The Book P. 67 Parallel Cases//IABR@RDM P. 87 The Free State of Amsterdam P. 99 Urban Century: How the World Becomes a City P. 111 Foaming at the Edge - Open City Master Class P. 113 Partner Program P. 117 Practical Information P. 121 Subsidizers and Partners P. 125 Credits P. 141 Colophon City 3 Preface gation of the favela’s inhabitants. She suggested that we refrain from trying The International Architecture Bien- to sell the idea that architects can turn nale Rotterdam (IABR) is an inter- Paraisópolis into paradise. Of course national urban research biennale I agreed. History has rarely been kind founded in 2001 with the conviction to those who want to build paradise that architecture is a public concern. on earth. So when the 4th IABR raises the issue of how architects and urban Architecture’s major challenge, in planners can concretely contribute to the eyes of the IABR, is to design and the design of coexistence, it has to be realize decent day-to-day living con- done with reserve. ditions for billions of people. With the theme Open City: Designing Coexis- Yet the question needs asking. tence, the 4th edition of the IABR places special emphasis on the social Whether exploding or shrinking, cit- aspect of this challenge: how can ies all over the world often tell tales architects and urban planners make of waste and neglect. -

2020 ESC Guidelines for the Management of Adult Congenital
ESC GUIDELINES European Heart Journal (2020) 00,1À83 doi:10.1093/eurheartj/ehaa554 2020 ESC Guidelines for the management of adult congenital heart disease The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD) Authors/Task Force Members: Helmut Baumgartner* (Chairperson) (Germany), Julie De Backer* (Chairperson) (Belgium), Sonya V. Babu-Narayan (United Kingdom), Werner Budts (Belgium), Massimo Chessa 1 (Italy), Gerhard-Paul Diller (Germany), Bernard Iung (France), Jolanda Kluin (Netherlands), Irene M. Lang (Austria), Folkert Meijboom (Netherlands), Philip Moons (Belgium), Barbara J. M. Mulder (Netherlands), Erwin Oechslin (Canada), Jolien W. Roos-Hesselink (Netherlands), Markus Schwerzmann (Switzerland), Lars Sondergaard (Denmark), Katja Zeppenfeld (Netherlands) Document reviewers: Sabine Ernst (CPG Review Coordinator) (United Kingdom), Magalie Ladouceur (CPG Review Coordinator) (France), Victor Aboyans (France), David Alexander (United Kingdom), Ruxandra Christodorescu (Romania), Domenico Corrado (Italy), Michele D’Alto (Italy), * Corresponding authors: Helmut Baumgartner, Department of Cardiology III: Adult Congenital and Valvular Heart Disease, University Hospital Muenster, Albert Schweitzer Campus 1, Building A1, D-48149, Muenster, Germany. Tel: þ49 251 83 46110, Fax: þ49 251 83 46109, E-mail: [email protected].