Phylogenetic Systematics, Morphological Evolution, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of 454-Ests from Huperzia Serrata And
Luo et al. BMC Plant Biology 2010, 10:209 http://www.biomedcentral.com/1471-2229/10/209 RESEARCH ARTICLE Open Access Comparison of 454-ESTs from Huperzia serrata and Phlegmariurus carinatus reveals putative genes involved in lycopodium alkaloid biosynthesis and developmental regulation Hongmei Luo1*, Ying Li1*, Chao Sun1, Qiong Wu1, Jingyuan Song1, Yongzhen Sun1, André Steinmetz2*, Shilin Chen1* Abstract Background: Plants of the Huperziaceae family, which comprise the two genera Huperzia and Phlegmariurus, produce various types of lycopodium alkaloids that are used to treat a number of human ailments, such as contusions, swellings and strains. Huperzine A, which belongs to the lycodine type of lycopodium alkaloids, has been used as an anti-Alzheimer’s disease drug candidate. Despite their medical importance, little genomic or transcriptomic data are available for the members of this family. We used massive parallel pyrosequencing on the Roche 454-GS FLX Titanium platform to generate a substantial EST dataset for Huperzia serrata (H. serrata) and Phlegmariurus carinatus (P. carinatus) as representative members of the Huperzia and Phlegmariurus genera, respectively. H. serrata and P. carinatus are important plants for research on the biosynthesis of lycopodium alkaloids. We focused on gene discovery in the areas of bioactive compound biosynthesis and transcriptional regulation as well as genetic marker detection in these species. Results: For H. serrata, 36,763 unique putative transcripts were generated from 140,930 reads totaling over 57,028,559 base pairs; for P. carinatus, 31,812 unique putative transcripts were generated from 79,920 reads totaling over 30,498,684 base pairs. Using BLASTX searches of public databases, 16,274 (44.3%) unique putative transcripts from H. -

General View of a Small Patch of Phylloglossum Plants. As Is Normal
. Fig. 1- General view of a small patch of Phylloglossum plants. As is normal in most populations there are many more sterile plants than fertile and a great range of sizes of plants are found. The 5-cent coin is about 2 cm in diameter and provides a convenient scale. 28 Phylloglossum Miniature Denizen of the North /. E. Braggins, Auckland Phylloglossum drummondii, first described by Kunze, a German botanist, in 1843, is a very small plant related to the lycopodiums or club mosses. In addition to New Zealand it is found in Western Australia, Tasmania, and Victoria. There is only one species of Phylloglossum, and because of its small size and habit of growing in low sedge and scrub it is not often detected. Furthermore it has a short growing season when it is above ground (May-June till September-October), and few botanists know it in the field. The description in Allan's Flora of New Zealand tells us that it is "... a plant up to 5 cm long, rarely more; leaves linear, acute, usually few, seldom more than 10, about 2 cm long". The stalk or peduncle of the fruiting part is described as 3-4 cm long, with a strobilus (sport-bearing part) about 7 mm long. The generic description having already said that the strobilus was terminal and roots scanty, we have some idea what the plant may look like, though words cannot convey adequately the appearance of this unusual little plant (Fig. 1). In New Zealand Phylloglossum is often regarded as a typical kauri and burnt-over scrubland plant. -

Department of the Interior Fish and Wildlife Service
Thursday, February 27, 2003 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Final Designation or Nondesignation of Critical Habitat for 95 Plant Species From the Islands of Kauai and Niihau, HI; Final Rule VerDate Jan<31>2003 13:12 Feb 26, 2003 Jkt 200001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\27FER2.SGM 27FER2 9116 Federal Register / Vol. 68, No. 39 / Thursday, February 27, 2003 / Rules and Regulations DEPARTMENT OF THE INTERIOR units designated for the 83 species. This FOR FURTHER INFORMATION CONTACT: Paul critical habitat designation requires the Henson, Field Supervisor, Pacific Fish and Wildlife Service Service to consult under section 7 of the Islands Office at the above address Act with regard to actions carried out, (telephone 808/541–3441; facsimile 50 CFR Part 17 funded, or authorized by a Federal 808/541–3470). agency. Section 4 of the Act requires us SUPPLEMENTARY INFORMATION: RIN 1018–AG71 to consider economic and other relevant impacts when specifying any particular Background Endangered and Threatened Wildlife area as critical habitat. This rule also and Plants; Final Designation or In the Lists of Endangered and determines that designating critical Nondesignation of Critical Habitat for Threatened Plants (50 CFR 17.12), there habitat would not be prudent for seven 95 Plant Species From the Islands of are 95 plant species that, at the time of species. We solicited data and Kauai and Niihau, HI listing, were reported from the islands comments from the public on all aspects of Kauai and/or Niihau (Table 1). -

Notes on Some Species of Diphasiastrum
Preslia, Praha, 47: 232 - 240, 1975 Notes on some species of Diphasiastrum Poznamky k n~kterym druhum rodu Dipha11iaatrum Josef Holub HOLUB J. (1975): Notes on some species of Diphasiastrum. - Preslia, Praha, 47: 232- 240. Taxonomic and nomenclatural problems of some species of Diphasiastrum HOLUB are discussed. A special attention is pa.id to the interspecies D. / X / issleri and D. / x / zei leri. Original plants of D. / x / issleri correspond to the combination D. alpinum - D. complanatum. Plants corresponding to the combination D. alpinum - D. tristachyum have been collected in the ~umava Mts. Some taxa described from the subarctic regions of Europe and North America are shown to belong most probably to the neglected interspecies D. / x / zeileri. Botanical I nstitute, Czechoslovak Academy of Sciences, 25~ 43 Prithonice, Czecho.,lovakia. INTRODUCTION This is a second part of my study of the new genus Diphasiastrum (HOLUB 1975), which could not be published in this journal in its entirety. Notes on taxonomy and nomenclature are selected from materials gathered originally for my "Catalogue of Czechoslovak vascular plants". With regard to the character of that work the present observations summarize the results of my own studies and suggests problems to be studied in the future. OBSERVATIONS f!.iphasiastrum alpinum (L.) HOLUB Two varieties have been described in this species (both under the name Lycopodium alpi nttm L.): var. thellungii HERTER from Switzerland and var. planiramulosum TAKEDA from Japan. Both these taxa, especially the first one, require a taxonomic revision; the possibility cannot be exclu<led that they are conspecific with D. / x / issleri. -

An Approach to the Problem of Taxonomy and Classification in the Study of Sporae Dispersae
AN APPROACH TO THE PROBLEM OF TAXONOMY AND CLASSIFICATION IN THE STUDY OF SPORAE DISPERSAE D. C. BHARDWAJ Birbal~i Institute of Palaeobotany, Lucknow ABSTRACT ( 1950 ) system, the American workers prefer The present state of disagreement among spore that of Schopf, Wilson and Bentall's ( 1944), workers with regard to the taxonomy and classi• in Germany, an amplification of Ibrahim's fication of Sporae dispersae has been reviewed. ( 1933) system is mostly in vogue and the The taxonomic categories for Sporae dispersae have Russian workers classify according to Nau• been defined and practicability of organ-genus concept over the form-genus concept for the circum• mova's (1937) system. This tendency to scription of spore taxa has been discussed. stick to one or the other of these systems, none of which excels the others, has uncons• ciously led to thwart the evolution of one such INTRODUCTION system of classification for the Sporae dis• persae which may be universally followed. Of late, in view of the greater applied use of DETAILEDand pollen,studyrecoveredof the dispersedfrom sedimen•spores Sporae dispersae in coal and oil prospecting, tary strata, dates back to Reinsch the need for a universally accepted standard ( 1881) when he described some of them from system of classification for these is being coals of the Carboniferous age. Thereafter, increasingly felt so that assimilation of data for several decades, these dispersed micro• from all over the world can be more easily remains attracted occasional attention of achieved. palaeobotanists -

Huperzine a from Huperzia Species—An Ethnopharmacolgical Review Xiaoqiang Ma A,B, Changheng Tan A, Dayuan Zhu A, David R
Huperzine A from Huperzia species—An ethnopharmacolgical review Xiaoqiang Ma a,b, Changheng Tan a, Dayuan Zhu a, David R. Gang b, Peigen Xiao c,∗ a State Key Laboratory of Drug Research, Institute of Materia Medica, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 201203, PR China b Department of Plant Sciences and BIO5 Institute, The University of Arizona, 303 Forbes Building, Tucson, AZ 85721-0036, USA c Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100094, PR China Abstract Huperzine A (HupA), isolated originally from a traditional Chinese medicine Qiang Ceng Ta, whole plant of Huperzia serrata (Thunb. ex Murray) Trev., a member of the Huperziaceae family, has attracted intense attention since its marked anticholinesterase activity was discovered by Chinese scientists. Several members of the Huperziaceae (Huperzia and Phlegmariurus species) have been used as medicines in China for contusions, strains, swellings, schizophrenia, myasthenia gravis and organophosphate poisoning. HupA has been marketed in China as a new drug for Alzheimer’s disease (AD) treatment and its derivative ZT-1 is being developed as anti-AD new drug candidate both in China and in Europe. A review of the chemistry, bioactivities, toxicology, clinical trials and natural resources of HupA source plants is presented. Keywords: Huperzine A; ZT-1; Alzheimer’s disease; Huperzia serrata; Huperziaceae; Drug discovery; Bioactivities; Clinical trials; Traditional Chinese -

(Lycopodiaceae) in the State of Veracruz, Mexico
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.8 (1): 114-137, 2015 Research article Distribution and conservation status of Phlegmariurus (Lycopodiaceae) in the state of Veracruz, Mexico Samaria Armenta-Montero1, César I. Carvajal-Hernández1, Edward A. Ellis1 and Thorsten Krömer1* 1Centro de Investigaciones Tropicales, Universidad Veracruzana, Casco de la Ex Hacienda Lucas Martín, Privada de Araucarias S/N. Col. Periodistas, C.P. 91019, Xalapa, Veracruz, Mexico *Corresponding author. Email: [email protected] Abstract The fern and lycophyte flora of Mexico contains 13 species in the genus Phlegmariurus (Lycopodiaceae; club moss family), of which nine are found in the state of Veracruz (P. cuernavacensis, P. dichotomus, P. linifolius, P. myrsinites, P. orizabae, P. pithyoides, P. pringlei, P. reflexus , P. taxifolius). They are located primarily in undisturbed areas of humid montane, pine-oak and tropical humid forests, which are all ecosystems threatened by deforestation and fragmentation. The objective of this study was to evaluate and understand the distribution and conservation status of species of this genus in the state of Veracruz, Mexico. Using Maxent, probability distributions were modeled based on 173 herbarium specimens (25% from recent collections by the authors and/or collaborators), considering factors such as climate, elevation and vegetation cover. Additionally, anthropogenic impacts on the original habitat of each species were analyzed in order to assign threatened categories based on IUCN classifications at regional levels. Results show that potential distributions are located in the montane regions of the central and southern parts of the state. All nine Phlegmariurus species in Veracruz were found to be in some category of risk, with P. -

Ecology and Distribution of Lycopodiaceae Mirbel in Malaysia
Blumea 54, 2009: 269–271 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476265 Ecology and distribution of Lycopodiaceae Mirbel in Malaysia G. Rusea1, K. Claysius1, S. Runi1,2, U. Joanes2, K.M. Haja Maideen3, A. Latiff 3 Key words Abstract This paper is the first account to discuss the distribution, ecology and habitats of the Lycopodiaceae in Malaysia. Lycopodiaceae are widely distributed throughout Malaysia with respect to altitudes and environmental distribution conditions but most abundantly found in hill forest and lower montane forest, terrestrial as well as epiphytic, in ecology shaded or semi-shaded places with relatively high humidity. Pahang in Peninsular Malaysia and Sabah in Borneo habitats have the highest species diversity in terms of the number of species collected. Lycopodiaceae Published on 30 October 2009 INTRODUCTION RESULTS AND DISCUSSION The Lycopodiaceae s.l. are an ancient (Correll 1956) and In Malaysia, the family comprises 32 species including 11 va- probably monophyletic family without close living relatives rieties that are found in various altitudes and vegetation types, and have a virtually cosmopolitan distribution (Øllgaard 1992). sometimes in a restricted area (Table 1). The estimated number of species ranges from approximately Lycopodiella cernua has the widest distribution in Malaysia 300 to more than 400 around the world (Wikström 2001). It and is most common on acid soils and occurs along forest consists of three genera namely Huperzia, Lycopodium and fringes, along roadside, hillsides and mountain slopes fol- Lycopodiella. Worldwide the estimated number of species for lowed by Huperzia carinata and H. pinifolia, which occur on both Lycopodium and Lycopodiella is about 40 (Wikström & tree branches. -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

Ecologia Mediterranea
ecologia mediterranea Revue Internationale d'Ecologie Méditerranéenne International Journal of Mediterranean Ecology Ecologie et conservation des mares temporaires méditerranéennes: l'exemple des mares de la Réserve Naturelle de Roque-Haute (Hérault, France) TOME 24· fascicule 2 • 1998 ISSN :0153-8756 REDACTEURS/ED/TORS COORDINATRICE / COORD/NATOR Frédéric MEDAIL Sophie BOUDEMAGHE Philip ROCHE Thierry TATONI TRESORIER / TREASURER Frédéric MAGNIN Jacques-Louis de BEAULIEU FONDATEUR/POUNDER Prof. Pierre QUEZEL COMITE DE LECTURE / ADV/SORY BOARD ARONSON J., CEFE CNRS, Montpellier LE FLOC'H E., CEFE CNRS, Montpellier BARBERO M., IMEP, Univ. Aix-Marseille III MARGARIS N. S., Univ. of the Aegan, Mytilène, Grèce BROCK M., Univ. ofNew England, Arrnidale, Australie OVALLE c., CSI Quilamapu, INIA, Chili CHEYLAN M., EPHE, Montpellier PEDROTTI F., Univ. degli Studi, Camerino, Italie DEBUSSCHE M., CEFE CNRS, Montpellier PLEGUEZUELOS 1. M., Univ. de Grenade, Espagne FADY 8., INRA, Avignon PONEL P., IMEP, CNRS, Marseille GOODFRIEND G. A., Carnegie Inst. W<H1ington, USA PRODON R., Labo. Arago, Univ. P. M. Curie, Paris VI GRILLAS P., Station Biologique Tour du Valat, Arles RICHARDSON D. M., Univ.Cape Town, Afrique du Sud GUIOT J., IMEP, CNRS, Marseille SANS F. X., Univ. de Barcelone, Espagne HOBBS R. 1., CSIRO, Midland, Australie SCHMIDA A., Hebrew Univ. of Jemsalem, Israël KRElTER S., ENSA-M INRA, Montpellier URBINATI C., Agripolis, Legnaro, Italie ecologia mediterranea Faculté des Sciences et Techniques de Saint Jérôme Institut Méditerranéen d'Ecologie et de Paléoécologie, case 461 13397 MARSEILLE Cedex 20 FRANCE Tél. : + 33491288976 - Fax: + 33491288051 E-mail: [email protected] URL: http://www.ecologia.fst.u-3mrs.fr Abonnements / Subscription Un an = deux numéros / one year = two issues: France: 400 F + 60 F de frais de port Europe : 400 F + 80 F de frais de port Amérique, Afrique, Asie: 400 F + 120 F de frais de port Tous les fascicules de la revue sont disponibles. -
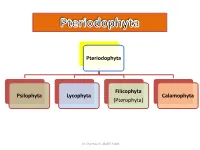
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

The Genus Huperzia (Lycopodiaceae) in the Azores and Madeira
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Biblioteca Digital do IPB Botanical Journal of the Linnean Society, 2008, 158, 522–533. With 15 figures The genus Huperzia (Lycopodiaceae) in the Azores and Madeira JOSÉ ANTONIO FERNÁNDEZ PRIETO1*, CARLOS AGUIAR2, EDUARDO DIAS3, MARÍA DE LOS ÁNGELES FERNÁNDEZ CASADO1 and JUAN HOMET1 1Área de Botánica, Departamento de Biología de Organismos y Sistemas, Universidad de Oviedo, 33006 Oviedo, Spain 2Área de Biologia, Escola Superior Agrária de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal 3Departamento de Ciências Agrárias, Universidade dos Açores, Campus de Angra, Terra-Chã, 9701-851 Angra do Heroísmo, Açores, Portugal Received 16 February 2005; accepted for publication 15 May 2008 The taxonomy and nomenclature of the genus Huperzia Bernh. in the Azores and Madeira have been reviewed. Plants collected in the Azores and Madeira were characterized morphologically. The independence between two endemic species common to Madeira and the Azores Islands – Huperzia suberecta (Lowe) Tardieu and Huperzia dentata (Herter) Holub – is clearly shown. A clear-cut morphological separation between these taxa and Huperzia selago (L.) Bernh. ex Schrank & Mart. of continental Europe is established. © 2008 The Linnean Society of London, Botanical Journal of the Linnean Society, 2008, 158, 522–533. ADDITIONAL KEYWORDS: bulbil – Huperzia dentata – Huperzia selago – Huperzia suberecta – nomen- clature – spore – stoma – taxonomy. INTRODUCTION Most authors have accepted this systematic treat- ment of Lycopodiaceae in Europe, including the Lycopodiaceae P.Beauv. ex Mirb. sensu lato is a genera Huperzia, Lycopodium, Diphasiastrum and family with a cosmopolitan distribution, consisting of Lycopodiella (Rothmaler, 1964, 1993; Villar, 1986).