New Combinations in the Vascular Flora of Finland, in the Genera Spinulum, Oxybasis and Potentilla
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lycopodium (Spp) L. Ground Pine
Kasey Hartz Natural Area Reference Sheet Lycopodium (spp) L. Ground pine. Lycopodiaceae (Club-moss Family) Blooming season: Not a flowering plant. Spores produced in July-September. Plant: Tree like in form, 10-25 cm tall. Horizontal stem, not a true rhizome; grows at one end only, and the opposite end dies, resulting in the plant slowly moving forward year by year (Billington 1952). Leaves: Evergreen, scale like. Flower: None, spores are produced on strobilus cones. Fruit: None, but what is formed are strobilus cones which are sessile, having no stalks. Usually 1-3 cones, 1.5-5.5 cm long, maturing July-September. Spores are sulphur yellow with a high oil content. Can be confused with: Many species of Lycopodium can be confused and hybrids may also be found. Geographic range: Type specimen location: State: Throughout. Regional: Newfoundland - Alaska, south to North Carolina and Indiana. Habitat: Local: Riparian. Regional: Moist woods. Common local companions: Pine, ferns, maples, and beech Kasey Hartz Natural Area Reference Sheet Lycopodium obscurum L. 2 Ground Pine Usages: Human: Because of the spores have a high oil content and are quite flammable, they were used as flash powder for the first photographic cameras(Harris 2003); for fireworks (Billington 1952); and to imitate lightning flashes for theatrical performances (Millspaugh 1892, 1974). Their oil content led to them being used by pharmacists in boxes of pills to prevent them sticking together (possibly a different species of Lycopodium). Medicinal uses in the past included treatments for gout, menstrual disorders, nervous disorders, fevers, and as a styptic and an emetic. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

Caryophyllales 2018 Instituto De Biología, UNAM September 17-23
Caryophyllales 2018 Instituto de Biología, UNAM September 17-23 LOCAL ORGANIZERS Hilda Flores-Olvera, Salvador Arias and Helga Ochoterena, IBUNAM ORGANIZING COMMITTEE Walter G. Berendsohn and Sabine von Mering, BGBM, Berlin, Germany Patricia Hernández-Ledesma, INECOL-Unidad Pátzcuaro, México Gilberto Ocampo, Universidad Autónoma de Aguascalientes, México Ivonne Sánchez del Pino, CICY, Centro de Investigación Científica de Yucatán, Mérida, Yucatán, México SCIENTIFIC COMMITTEE Thomas Borsch, BGBM, Germany Fernando O. Zuloaga, Instituto de Botánica Darwinion, Argentina Victor Sánchez Cordero, IBUNAM, México Cornelia Klak, Bolus Herbarium, Department of Biological Sciences, University of Cape Town, South Africa Hossein Akhani, Department of Plant Sciences, School of Biology, College of Science, University of Tehran, Iran Alexander P. Sukhorukov, Moscow State University, Russia Michael J. Moore, Oberlin College, USA Compilation: Helga Ochoterena / Graphic Design: Julio C. Montero, Diana Martínez GENERAL PROGRAM . 4 MONDAY Monday’s Program . 7 Monday’s Abstracts . 9 TUESDAY Tuesday ‘s Program . 16 Tuesday’s Abstracts . 19 WEDNESDAY Wednesday’s Program . 32 Wednesday’s Abstracs . 35 POSTERS Posters’ Abstracts . 47 WORKSHOPS Workshop 1 . 61 Workshop 2 . 62 PARTICIPANTS . 63 GENERAL INFORMATION . 66 4 Caryophyllales 2018 Caryophyllales General program Monday 17 Tuesday 18 Wednesday 19 Thursday 20 Friday 21 Saturday 22 Sunday 23 Workshop 1 Workshop 2 9:00-10:00 Key note talks Walter G. Michael J. Moore, Berendsohn, Sabine Ya Yang, Diego F. Registration -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Effects of Lycopodium Clavatum and Equisetum Arvense Extracts from Western Romania
Romanian Biotechnological Letters Vol. , No. x, Copyright © 2016 University of Bucharest Printed in Romania. All rights reserved ORIGINAL PAPER Effects of Lycopodium clavatum and equisetum arvense extracts from western Romania Received for publication, July, 07, 2014 Accepted, October, 13, 2015 MARIA SUCIU1, FELIX AUREL MIC1, LUCIAN BARBU-TUDORAN2, VASILE MUNTEAN2, ALEXANDRA TEODORA GRUIA3,* 1University of Medicine and Pharmacy “Victor Babes”, Department of Functional Sciences, Timisoara, 2, Eftimie Murgu Sq., Timisoara, 300041, Timis County, Romania 2Babes-Bolyai University, Biology and Geology Department, 5-7 Clinicilor Str., Cluj-Napoca, 400084, Cluj County, Romania. 3Emergency Clinical County Hospital Timisoara, Regional Centre for Transplant Immunology Department, 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. *Address for correspondence to: [email protected], 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. Abbreviations: ALT–alanin transaminases, AST–aspartate transaminases, GC-MS–gas chromatograph coupled with mass spectrometry. Abstract Plants have always excited interest because of their active principles that could be a source of healing in various affections. The aim of this study was to demonstrate that the hepatoprotective and antimicrobial effects of Lycopodium clavatum and Equisetum arvense from the Western parts of Romania (Arad County) are not as pronounced as described in literature, against xenobiotic intoxication or microbial infection. To identify the plants active compounds, -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Notes on Some Species of Diphasiastrum
Preslia, Praha, 47: 232 - 240, 1975 Notes on some species of Diphasiastrum Poznamky k n~kterym druhum rodu Dipha11iaatrum Josef Holub HOLUB J. (1975): Notes on some species of Diphasiastrum. - Preslia, Praha, 47: 232- 240. Taxonomic and nomenclatural problems of some species of Diphasiastrum HOLUB are discussed. A special attention is pa.id to the interspecies D. / X / issleri and D. / x / zei leri. Original plants of D. / x / issleri correspond to the combination D. alpinum - D. complanatum. Plants corresponding to the combination D. alpinum - D. tristachyum have been collected in the ~umava Mts. Some taxa described from the subarctic regions of Europe and North America are shown to belong most probably to the neglected interspecies D. / x / zeileri. Botanical I nstitute, Czechoslovak Academy of Sciences, 25~ 43 Prithonice, Czecho.,lovakia. INTRODUCTION This is a second part of my study of the new genus Diphasiastrum (HOLUB 1975), which could not be published in this journal in its entirety. Notes on taxonomy and nomenclature are selected from materials gathered originally for my "Catalogue of Czechoslovak vascular plants". With regard to the character of that work the present observations summarize the results of my own studies and suggests problems to be studied in the future. OBSERVATIONS f!.iphasiastrum alpinum (L.) HOLUB Two varieties have been described in this species (both under the name Lycopodium alpi nttm L.): var. thellungii HERTER from Switzerland and var. planiramulosum TAKEDA from Japan. Both these taxa, especially the first one, require a taxonomic revision; the possibility cannot be exclu<led that they are conspecific with D. / x / issleri. -

(Lycopodiaceae) in the State of Veracruz, Mexico
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.8 (1): 114-137, 2015 Research article Distribution and conservation status of Phlegmariurus (Lycopodiaceae) in the state of Veracruz, Mexico Samaria Armenta-Montero1, César I. Carvajal-Hernández1, Edward A. Ellis1 and Thorsten Krömer1* 1Centro de Investigaciones Tropicales, Universidad Veracruzana, Casco de la Ex Hacienda Lucas Martín, Privada de Araucarias S/N. Col. Periodistas, C.P. 91019, Xalapa, Veracruz, Mexico *Corresponding author. Email: [email protected] Abstract The fern and lycophyte flora of Mexico contains 13 species in the genus Phlegmariurus (Lycopodiaceae; club moss family), of which nine are found in the state of Veracruz (P. cuernavacensis, P. dichotomus, P. linifolius, P. myrsinites, P. orizabae, P. pithyoides, P. pringlei, P. reflexus , P. taxifolius). They are located primarily in undisturbed areas of humid montane, pine-oak and tropical humid forests, which are all ecosystems threatened by deforestation and fragmentation. The objective of this study was to evaluate and understand the distribution and conservation status of species of this genus in the state of Veracruz, Mexico. Using Maxent, probability distributions were modeled based on 173 herbarium specimens (25% from recent collections by the authors and/or collaborators), considering factors such as climate, elevation and vegetation cover. Additionally, anthropogenic impacts on the original habitat of each species were analyzed in order to assign threatened categories based on IUCN classifications at regional levels. Results show that potential distributions are located in the montane regions of the central and southern parts of the state. All nine Phlegmariurus species in Veracruz were found to be in some category of risk, with P. -

Ecology and Distribution of Lycopodiaceae Mirbel in Malaysia
Blumea 54, 2009: 269–271 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476265 Ecology and distribution of Lycopodiaceae Mirbel in Malaysia G. Rusea1, K. Claysius1, S. Runi1,2, U. Joanes2, K.M. Haja Maideen3, A. Latiff 3 Key words Abstract This paper is the first account to discuss the distribution, ecology and habitats of the Lycopodiaceae in Malaysia. Lycopodiaceae are widely distributed throughout Malaysia with respect to altitudes and environmental distribution conditions but most abundantly found in hill forest and lower montane forest, terrestrial as well as epiphytic, in ecology shaded or semi-shaded places with relatively high humidity. Pahang in Peninsular Malaysia and Sabah in Borneo habitats have the highest species diversity in terms of the number of species collected. Lycopodiaceae Published on 30 October 2009 INTRODUCTION RESULTS AND DISCUSSION The Lycopodiaceae s.l. are an ancient (Correll 1956) and In Malaysia, the family comprises 32 species including 11 va- probably monophyletic family without close living relatives rieties that are found in various altitudes and vegetation types, and have a virtually cosmopolitan distribution (Øllgaard 1992). sometimes in a restricted area (Table 1). The estimated number of species ranges from approximately Lycopodiella cernua has the widest distribution in Malaysia 300 to more than 400 around the world (Wikström 2001). It and is most common on acid soils and occurs along forest consists of three genera namely Huperzia, Lycopodium and fringes, along roadside, hillsides and mountain slopes fol- Lycopodiella. Worldwide the estimated number of species for lowed by Huperzia carinata and H. pinifolia, which occur on both Lycopodium and Lycopodiella is about 40 (Wikström & tree branches. -

Ecophysiology of Four Co-Occurring Lycophyte Species: an Investigation of Functional Convergence
Research Article Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence Jacqlynn Zier, Bryce Belanger, Genevieve Trahan and James E. Watkins* Department of Biology, Colgate University, Hamilton, NY 13346, USA Received: 22 June 2015; Accepted: 7 November 2015; Published: 24 November 2015 Associate Editor: Tim J. Brodribb Citation: Zier J, Belanger B, Trahan G, Watkins JE. 2015. Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence. AoB PLANTS 7: plv137; doi:10.1093/aobpla/plv137 Abstract. Lycophytes are the most early divergent extant lineage of vascular land plants. The group has a broad global distribution ranging from tundra to tropical forests and can make up an important component of temperate northeast US forests. We know very little about the in situ ecophysiology of this group and apparently no study has eval- uated if lycophytes conform to functional patterns expected by the leaf economics spectrum hypothesis. To determine factors influencing photosynthetic capacity (Amax), we analysed several physiological traits related to photosynthesis to include stomatal, nutrient, vascular traits, and patterns of biomass distribution in four coexisting temperate lycophyte species: Lycopodium clavatum, Spinulum annotinum, Diphasiastrum digitatum and Dendrolycopodium dendroi- deum. We found no difference in maximum photosynthetic rates across species, yet wide variation in other traits. We also found that Amax was not related to leaf nitrogen concentration and is more tied to stomatal conductance, suggestive of a fundamentally different sets of constraints on photosynthesis in these lycophyte taxa compared with ferns and seed plants. These findings complement the hydropassive model of stomatal control in lycophytes and may reflect canaliza- tion of function in this group. -
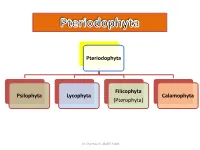
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Natural Community and Plant Inventory of Grass River Natural
Natural Community Delineation and Floristic Quality Assessments of Grass River Natural Area, Antrim County, Michigan Prepared by: Rachel Hackett, Phyllis Higman, and Liana May Michigan Natural Features Inventory PO Box 13036 Lansing, MI 48901-3036 For: Grass River Natural Area 6500 Alden Hwy, Bellaire, MI 49615 December 31, 2017 Report No. 2017-12 Funding for this project was provided by the Grass River Natural Area through a grant from the Grand Traverse Regional Community Foundation. Suggested Citation: Hackett, R.A., P. Higman, and L. May. 2017. Natural Community Delineation and Floristic Quality Assessments of Grass River Natural Area, Antrim County, Michigan. Michigan Natural Features Inventory, Report No. 2017-12, Lansing, MI. 64 pp. Appendices: 62 pp. Copyright 2017 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientations, marital status, or family status. Cover photographs: Utricularia cornuta in northern fen, MI #1A, Antrim Co., Mich., June 20, 2017; Sarracenia purpurea in rich conifer swamp, DELANGE #1B, Antrim Co., MI, June 23, 2017. All photographs in report by R.A. Hackett unless otherwise noted. Table of Contents Executive Summary ........................................................................................................................ 1 Introduction ....................................................................................................................................