MR Imaging of the Temporal Stem: Anatomic Dissection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FUS-Mediated Functional Neuromodulation for Neurophysiologic Assessment in a Large Animal Model Wonhye Lee1*, Hyungmin Kim2, Stephanie D
Lee et al. Journal of Therapeutic Ultrasound 2015, 3(Suppl 1):O23 http://www.jtultrasound.com/content/3/S1/O23 ORALPRESENTATION Open Access FUS-mediated functional neuromodulation for neurophysiologic assessment in a large animal model Wonhye Lee1*, Hyungmin Kim2, Stephanie D. Lee1, Michael Y. Park1, Seung-Schik Yoo1 From Current and Future Applications of Focused Ultrasound 2014. 4th International Symposium Washington, D.C, USA. 12-16 October 2014 Background/introduction element FUS transducer with radius-of-curvature of Focused ultrasound (FUS) is gaining momentum as a 7 cm) was transcranially delivered to the unilateral sen- new modality of non-invasive neuromodulation of regio- sorimotor cortex, the optic radiation (WM tract) as well as nal brain activity, with both stimulatory and suppressive the visual cortex. An acoustic intensity of 1.4–15.5 W/cm2 potentials. The utilization of the method has largely Isppa, tone-burst-duration of 1 ms, pulse-repetition fre- been demonstrated in small animals. Considering the quency of 500 Hz (i.e. duty cycle of 50%), sonication dura- small size of the acoustic focus, having a diameter of tion of 300 ms, were used for the stimulation. A batch of only a few millimeters, FUS insonification to a larger continuous sonication ranging from 50 to 150 ms in dura- animal’s brain is conducive to examining the region- tion were also given. Evoked electromyogram responses specific neuromodulatory effects on discrete anatomical from the hind legs and electroencephalogram from the Fz areas, including the white matter (WM) tracts. The and Oz-equivalent sites were measured. The histology of study involving large animals would also establish preli- the extracted brain tissue (within one week and 2 months minary safety data prior to its translational research in post-sonication) was obtained. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
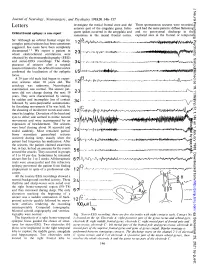
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -

A Bilateral Cortico-Striate Projection
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.1.71 on 1 February 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 71 A bilateral cortico-striate projection J. B. CARMAN, W. M. COWAN, T. P. S. POWELL, AND K. E. WEBSTER From the Departments of Anatomy, University of Oxford, and University College, London During the course of studies on the projection of the ined, and evidence for a bilateral projection has been cerebral cortex upon the striatum in the rabbit found in 20 animals. The evidence for this projec- (Carman, Cowan, and Powell, 1963) and the cat tion depends upon the collective findings in several (Webster, 1964) degeneration was seen bilaterally in brains, but only a few typical examples will be Nauta preparations of the striatum in some, but not described in full. The findings in the remaining all, animals. For two main reasons this observation experiments will be summarized in composite was not included in the earlier study. First, because figures. of the difficulty of interpreting any findings of Experiment R30 is representative of the rabbit bilateral degeneration in silver preparations, and, brains in which a projection to the contralateral particularly as it is well known that the striatum striatum was found after a lesion involving the sen- commonly shows pseudo-degeneration, it was im- sori-motor cortex. The cortical damage in this brain perative to exclude this possibility by the prepara- is in the form of a broad strip along the dorsal guest. Protected by copyright. tion of further material using both the frozen and surface of the hemisphere from just behind the paraffin Nauta methods. -

Revista Brasileira De Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012
Rev Bras Psiquiatr. 2012;34:101-111 Revista Brasileira de Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012 REVIEW ARTICLE Neuroimaging in specific phobia disorder: a systematic review of the literature Ila M.P. Linares,1 Clarissa Trzesniak,1 Marcos Hortes N. Chagas,1 Jaime E. C. Hallak,1 Antonio E. Nardi,2 José Alexandre S. Crippa1 ¹ Department of Neuroscience and Behavior of the Ribeirão Preto Medical School, Universidade de São Paulo (FMRP-USP). INCT Translational Medicine (CNPq). São Paulo, Brazil 2 Panic & Respiration Laboratory. Institute of Psychiatry, Universidade Federal do Rio de Janeiro (UFRJ). INCT Translational Medicine (CNPq). Rio de Janeiro, Brazil Received on August 03, 2011; accepted on October 12, 2011 DESCRIPTORS Abstract Neuroimaging; Objective: Specific phobia (SP) is characterized by irrational fear associated with avoidance of Specific Phobia; specific stimuli. In recent years, neuroimaging techniques have been used in an attempt to better Review; understand the neurobiology of anxiety disorders. The objective of this study was to perform a Anxiety Disorder; systematic review of articles that used neuroimaging techniques to study SP. Method: A literature Phobia. search was conducted through electronic databases, using the keywords: imaging, neuroimaging, PET, spectroscopy, functional magnetic resonance, structural magnetic resonance, SPECT, MRI, DTI, and tractography, combined with simple phobia and specific phobia. One-hundred fifteen articles were found, of which 38 were selected for the present review. From these, 24 used fMRI, 11 used PET, 1 used SPECT, 2 used structural MRI, and none used spectroscopy. Result: The search showed that studies in this area were published recently and that the neuroanatomic substrate of SP has not yet been consolidated. -

Rhesus Monkey Brain Atlas Subcortical Gray Structures
Rhesus Monkey Brain Atlas: Subcortical Gray Structures Manual Tracing for Hippocampus, Amygdala, Caudate, and Putamen Overview of Tracing Guidelines A) Tracing is done in a combination of the three orthogonal planes, as specified in the detailed methods that follow. B) Each region of interest was originally defined in the right hemisphere. The labels were then reflected onto the left hemisphere and all borders checked and adjusted manually when necessary. C) For the initial parcellation, the user used the “paint over function” of IRIS/SNAP on the T1 template of the atlas. I. Hippocampus Major Boundaries Superior boundary is the lateral ventricle/temporal horn in the majority of slices. At its most lateral extent (subiculum) the superior boundary is white matter. The inferior boundary is white matter. The anterior boundary is the lateral ventricle/temporal horn and the amygdala; the posterior boundary is lateral ventricle or white matter. The medial boundary is CSF at the center of the brain in all but the most posterior slices (where the medial boundary is white matter). The lateral boundary is white matter. The hippocampal trace includes dentate gyrus, the CA3 through CA1 regions of the hippocamopus, subiculum, parasubiculum, and presubiculum. Tracing A) Tracing is done primarily in the sagittal plane, working lateral to medial a. Locate the most lateral extent of the subiculum, which is bounded on all sides by white matter, and trace. b. As you page medially, tracing the hippocampus in each slice, the superior, anterior, and posterior boundaries of the hippocampus become the lateral ventricle/temporal horn. c. Even further medially, the anterior boundary becomes amygdala and the posterior boundary white matter. -

Uncinate Fasciculus Findings in Schizophrenia: a Magnetic Resonance Diffusion Tensor Imaging Study
Article Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study Marek Kubicki, M.D., Ph.D. Objective: Disruptions in connectivity prominent white matter tract connecting between the frontal and temporal lobes temporal and frontal brain regions, in 15 Carl-Fredrik Westin, Ph.D. may explain some of the symptoms ob- patients with chronic schizophrenia and served in schizophrenia. Conventional 18 normal comparison subjects. A 1.5-T GE Stephan E. Maier, M.D., Ph.D. magnetic resonance imaging (MRI) stud- Echospeed system was used to acquire 4- ies, however, have not shown compelling mm-thick coronal line-scan diffusion ten- evidence for white matter abnormalities, sor images. Maps of the fractional anisot- Melissa Frumin, M.D. because white matter fiber tracts cannot ropy were generated to quantify the water be visualized by conventional MRI. Diffu- diffusion within the uncinate fasciculus. Paul G. Nestor, Ph.D. sion tensor imaging is a relatively new technique that can detect subtle white Results: Findings revealed a group-by- Dean F. Salisbury, Ph.D. matter abnormalities in vivo by assessing side interaction for fractional anisotropy the degree to which directionally orga- and for uncinate fasciculus area, derived from automatic segmentation. The pa- Ron Kikinis, M.D. nized fibers have lost their normal integ- rity. The first three diffusion tensor imag- tients with schizophrenia showed a lack of ing studies in schizophrenia showed lower normal left-greater-than-right asymmetry Ferenc A. Jolesz, M.D. anisotropic diffusion, relative to compari- seen in the comparison subjects. son subjects, in whole-brain white matter, Robert W. -

The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1935 The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat James K. L. Choy Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Anatomy Commons Recommended Citation Choy, James K. L., "The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat" (1935). Master's Theses. 22. https://ecommons.luc.edu/luc_theses/22 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1935 James K. L. Choy LOYOLA UNIVERSITY SCHOOl, OF MEDICINE THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. A THESIS SUBMITTED TO THE FACULTY of the GRADUATE SCHOOL of LOYOLA UNIVERSITY IN CANDIDACY FOR THE DEGREE OF MASTER OF SCIENCE by James K.L. Choy, B.S.M. 1935 THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. I. PREFACE Before entering upon a discussion of the problem itself, I would lil{e to take this opportunity to acknowledge the assis tance and encouragement I received in the preparation of this paper. To Dr. R. M. Strong, who suggested the problem, I am deeply obligated for his encouragement, practical guidance, and helpful suggestions in the procedure of this work. -

Integrity of the Uncinate Fasciculus Is Associated with the Onset
Li et al. Translational Psychiatry (2021) 11:111 https://doi.org/10.1038/s41398-021-01222-z Translational Psychiatry ARTICLE Open Access Integrity of the uncinate fasciculus is associated with the onset of bipolar disorder: a 6-year followed-up study Xiaoyue Li1,2, Weicong Lu1,2,RuoxiZhang1,2, Wenjin Zou1,2, Yanling Gao1,2, Kun Chen1,2,Suk-YuYau3,RobinShao1,4, Roger S. McIntyre5,6,7,GuiyunXu1,2,Kwok-FaiSo 1,2,8 and Kangguang Lin 1,2 Abstract Patients with Bipolar Disorder (BD) are associated with aberrant uncinate fasciculus (UF) that connects amygdala- ventral prefrontal cortex (vPFC) system, but the casual relationship is still uncertain. The research aimed to investigate the integrity of UF among offspring of patients with BD and investigate its potential causal association with subsequent declaration of BD. The fractional anisotropy (FA) and mean diffusivity (MD) of UF were compared in asymptomatic offspring (AO, n = 46) and symptomatic offspring (SO, n = 45) with a parent with BD, and age-matched healthy controls (HCs, n = 35). Logistic regressions were performed to assess the predictive effect of UF integrity on the onset of BD. The three groups did not differ at baseline in terms of FA and MD of the UF. Nine out of 45 SO developed BD over a follow-up period of 6 years, and the right UF FA predicted the onset of BD (p = 0.038, OR = 0.212, 95% CI = 0.049–0.917). The ROC curve revealed that the right UF FA predicted BD onset (area-under-curve = 0.859) with sensitivity of 88.9% and specificity of 77.3%. -

Embryology, Anatomy, and Physiology of the Afferent Visual Pathway
CHAPTER 1 Embryology, Anatomy, and Physiology of the Afferent Visual Pathway Joseph F. Rizzo III RETINA Physiology Embryology of the Eye and Retina Blood Supply Basic Anatomy and Physiology POSTGENICULATE VISUAL SENSORY PATHWAYS Overview of Retinal Outflow: Parallel Pathways Embryology OPTIC NERVE Anatomy of the Optic Radiations Embryology Blood Supply General Anatomy CORTICAL VISUAL AREAS Optic Nerve Blood Supply Cortical Area V1 Optic Nerve Sheaths Cortical Area V2 Optic Nerve Axons Cortical Areas V3 and V3A OPTIC CHIASM Dorsal and Ventral Visual Streams Embryology Cortical Area V5 Gross Anatomy of the Chiasm and Perichiasmal Region Cortical Area V4 Organization of Nerve Fibers within the Optic Chiasm Area TE Blood Supply Cortical Area V6 OPTIC TRACT OTHER CEREBRAL AREASCONTRIBUTING TO VISUAL LATERAL GENICULATE NUCLEUSPERCEPTION Anatomic and Functional Organization The brain devotes more cells and connections to vision lular, magnocellular, and koniocellular pathways—each of than any other sense or motor function. This chapter presents which contributes to visual processing at the primary visual an overview of the development, anatomy, and physiology cortex. Beyond the primary visual cortex, two streams of of this extremely complex but fascinating system. Of neces- information flow develop: the dorsal stream, primarily for sity, the subject matter is greatly abridged, although special detection of where objects are and for motion perception, attention is given to principles that relate to clinical neuro- and the ventral stream, primarily for detection of what ophthalmology. objects are (including their color, depth, and form). At Light initiates a cascade of cellular responses in the retina every level of the visual system, however, information that begins as a slow, graded response of the photoreceptors among these ‘‘parallel’’ pathways is shared by intercellular, and transforms into a volley of coordinated action potentials thalamic-cortical, and intercortical connections. -

Marrow Stromal Cells Migrate Throughout Forebrain and Cerebellum, and They Differentiate Into Astrocytes After Injection Into Neonatal Mouse Brains
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 10711–10716, September 1999 Cell Biology Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains GENE C. KOPEN,DARWIN J. PROCKOP, AND DONALD G. PHINNEY* Center for Gene Therapy, MCP Hahnemann University, 245 North 15th Street, Philadelphia, PA 19102-1192 Contributed by Darwin J. Prockop, July 16, 1999 ABSTRACT Stem cells are a valuable resource for treat- cell lineages after transplantation into the hematopoietic ing disease, but limited access to stem cells from tissues such system of irradiated hosts (11). Together, these findings sug- as brain restricts their utility. Here, we injected marrow gest that it may be possible to reconstitute a tissue by using stromal cells (MSCs) into the lateral ventricle of neonatal stem cells from a separate dermal origin. To determine mice and asked whether these multipotential mesenchymal whether mesenchymal stem cells can adopt neural cell fates, we progenitors from bone marrow can adopt neural cell fates injected a purified population of murine MSCs into the lateral when exposed to the brain microenvironment. By 12 days ventricles of neonatal mice and examined the fate of these cells postinjection, MSCs migrated throughout the forebrain and by immunohistochemistry. cerebellum without disruption to the host brain architecture. Some MSCs within the striatum and the molecular layer of the MATERIALS AND METHODS hippocampus expressed glial fibrillary acidic protein and, therefore, differentiated into mature astrocytes. MSCs also Isolation and Purification of MSCs. Murine MSC cultures populated neuron rich regions including the Islands of were established from the bone marrow of FVB͞N mice (The Calleja, the olfactory bulb, and the internal granular layer of Jackson Laboratory) and were cultured for 7 days (12).