Chagas Disease in Nicaragua
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infected Areas As at 13 April 1995 Zones Infectées Au 13 Avril 1995 for Criteria Used in Compiling This Hsc, See No
WEEKLY EPIDEMIOLOGICAL RECORD, No. 1 5 ,1 4 APRIL 1995 • RELEVÉ ÉPIDÉMIOLOGIQUE HEBDOMADAIRE, N* 1 5 ,1 4 AVRIL 1995 to Ae vaccination and die dry weather which reduced the vaccination et l’arrivée de la saison sèche qui a réduit la densité vector density. However, dine could be an increase in vectorielle. Toutefois, il se peut que l’on assiste à une augmenta yellow fever in April 1995 when the rains start, particularly tion du nombre de cas de fièvre jaune en avril 1995, quand in villages not affected by the present epidemic. The commenceront les pluies, notamment dans les villages non touchés following action should be taken to prevent this: par l’épidémie actuelle. Pour l’éviter, il conviendrait de prendre les mesures suivantes: (1) Yellow-fever vaccination must continue. This 1) Il feut étendre la vaccination anti-amarile. Cette stratégie strategy should be actively pursued from village to doit être activement poursuivie de village en village pour village to ensure the inclusion of young children. que les jeunes e n f a n t s soient vaccinés. Des fiches de vacci Immunization cards should be given to all vaccin nation doivent être remises à toutes les personnes vacci ated persons. nées. (2) Early case detection and reporting are extremely 2) Le dépistage précoce et la notification sont extrêmement important The health facilities are not familiar importants. Les services de santé ne sont guère au courant with the reporting system, and training on disease du système de notification, et il faut préparer à la surveil surveillance and notification must be given to lance et à la notification des maladies le personnel des health facility workers from the area and from adja services de santé de la zone ainsi que des LGA limitrophes cent yellow-fever endemic LG As. -

Leveraging Municipal Government Support for Agricultural Value Chains in Nicaragua
LEVERAGING MUNICIPAL GOVERNMENT SUPPORT FOR AGRICULTURAL VALUE CHAINS IN NICARAGUA Leveraging Municipal Government Support for Agricultural Value Chains in Nicaragua Jefferson Shriver and Jorge Manuel Brenes Abdalah HARNESSING THE POWER OF SILC TO DRIVE AGROENTERPRISE DEVELOPMENT IN GHANA Sections of this publication may be copied or adapted to meet local needs without permission from Catholic Relief Services, provided that the parts copied are distributed for free or at cost—not for profit. For any commercial reproduction, please obtain permission from: Catholic Relief Services 228 W. Lexington St. Baltimore, MD 21201 (Tel) 410-625-2220 Attn: PQSD Microfinance Unit (Fax) 410-234-3178 Attn: PQSD Microfinance Unit Email: [email protected] Main CRS website: www.crs.org CRS technical publications: www.crsprogramquality.org Printed in the United States of America Copyright © 2012 Catholic Relief Services ISBN-13: 978-1-61492-111-0 ISBN-10: 1-614-9211-3 HARNESSING THE POWER OF SILC TO DRIVE AGROENTERPRISE DEVELOPMENT IN GHANA Leveraging Municipal Government Support for Agricultural Value Chains in Nicaragua Attracting Private Sector Investment to Rural and Agricultural Markets Jefferson Shriver and Jorge Manuel Brenes Abdalah June 2012 CRACKING THE NUT—ATTRACTING PRIVATE SECTOR INVESTMENT TO RURAL AND AGRICULTURAL MARKETS ACORDAR Project Summary The Alliance to Create Rural Business Opportunities through Agroenterprise Relationships (ACORDAR) is a USAID-sponsored, public-private sector, five- year initiative (2007–12) directly benefitting 7,000 smallholders, organized in 107 cooperatives in 50 municipalities of Nicaragua. Total project investments amount to US$53 million. Project objectives are to assist small producers to increase their income, ensure ongoing employment, and strengthen their market-access capacities, through agricultural value chain development, in alliance with municipal governments, and private sector partnership in Nicaragua. -

Improved Pastures Support Early Indicators of Soil Restoration in Low-Input Agroecosystems of Nicaragua
Environmental Management (2019) 64:201–212 https://doi.org/10.1007/s00267-019-01181-8 Improved Pastures Support Early Indicators of Soil Restoration in Low-input Agroecosystems of Nicaragua 1 1 2,3 4 5 Emily Webster ● Amélie C. M. Gaudin ● Mirjam Pulleman ● Pablo Siles ● Steven J. Fonte Received: 28 February 2018 / Accepted: 7 June 2019 / Published online: 18 June 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Pasture degradation hinders livestock production and ecosystem services that support rural smallholder communities throughout Latin America. Silvopastoral systems, with improved pasture cultivars (especially Brachiaria spp.) and multipurpose trees, offer a promising strategy to restore soils and improve livelihoods in the region. However, studies evaluating the impact of such systems on pasture productivity and soil health under realistic smallholder constraints are lacking. We evaluated the impact of improved pasture grass and tree establishment on a suite of soil health indicators in actively grazed, low-input, farmer-managed silvopastoral systems. In August 2013, paired pasture treatments (improved grass with trees vs. traditional pastures) were established on nine farms with similar land-use histories near Matagalpa, 1234567890();,: 1234567890();,: Nicaragua. On each farm, one treatment was left as traditional pasture with naturalized grass (Hyparrhenia rufa), while the adjacent treatment was sown with the improved grass (Brachiaria brizantha cv. Marandu) and planted with tree saplings without fertilizer. In August 2015, we measured standing biomass and a suite of chemical, biological, and physical soil health variables. Improved silvopastoral systems with B. brizantha produced more standing grass biomass and supported higher levels of earthworm populations and permanganate oxidizable carbon (POXC) compared to the traditional control. -

Nicaragua Expansion and Strengthening Of
PUBLIC SIMULTANEOUS DISCLOSURE DOCUMENT OF THE INTER-AMERICAN DEVELOPMENT BANK NICARAGUA EXPANSION AND STRENGTHENING OF NICARAGUA’S ELECTRICITY TRANSMISSION SYSTEM (NI-L1091) LOAN PROPOSAL This document was prepared by the project team consisting of: Héctor Baldivieso (ENE/CNI), Project Team Leader; Arnaldo Vieira de Carvalho (INE/ENE), Project Team Co-leader; Alberto Levy (INE/ENE); Carlos Trujillo (INE/ENE); Carlos Hinestrosa (INE/ENE); Stephanie Suber (INE/ENE); Juan Carlos Lazo (FMP/CNI); Santiago Castillo (FMP/CNI); María Cristina Landázuri (LEG/SGO); Denis Corrales (VPS/ESG); and Alma Reyna Selva (CID/CNI). This document is being released to the public and distributed to the Bank’s Board of Executive Directors simultaneously. This document has not been approved by the Board. Should the Board approve the document with amendments, a revised version will be made available to the public, thus superseding and replacing the original version. CONTENTS PROJECT SUMMARY I. DESCRIPTION AND RESULTS MONITORING ................................................................ 1 A. Background, problem to be addressed, and rationale ................................... 1 B. Objectives, components, and cost ................................................................ 8 C. Key results indicators ................................................................................. 10 II. FINANCING STRUCTURE AND MAIN RISKS ............................................................... 10 A. Financing instruments ............................................................................... -

Nicaragua: 14 Killed in Anti-Tank Mine Explosion in Jinotega Department Deborah Tyroler
University of New Mexico UNM Digital Repository NotiCen Latin America Digital Beat (LADB) 8-23-1991 Nicaragua: 14 Killed In Anti-tank Mine Explosion In Jinotega Department Deborah Tyroler Follow this and additional works at: https://digitalrepository.unm.edu/noticen Recommended Citation Tyroler, Deborah. "Nicaragua: 14 Killed In Anti-tank Mine Explosion In Jinotega Department." (1991). https://digitalrepository.unm.edu/noticen/5954 This Article is brought to you for free and open access by the Latin America Digital Beat (LADB) at UNM Digital Repository. It has been accepted for inclusion in NotiCen by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. LADB Article Id: 065364 ISSN: 1089-1560 Nicaragua: 14 Killed In Anti-tank Mine Explosion In Jinotega Department by Deborah Tyroler Category/Department: General Published: Friday, August 23, 1991 In an Aug. 21 broadcast, Radio Ya reported that 12 recontras under the command of "Indomable" were killed and an indeterminate number wounded when the truck they were traveling in passed over an anti-tank mine, and went over a cliff. The incident occurred near Pita del Carmen, Jinotega department. During an Interior Ministry briefing on Aug. 22, National Police chief Rene Vivas said 14 persons were killed, and seven were recontras. According to Vivas, the mine had intentionally been placed in the roadway, and an investigation was underway. Vivas said seven AK-47 rifles and other materiel were discovered near the site of mine explosion. He added that one of the dead was identified as "El Chino #2," a member of another recontra group headed by "El Tigrillo." Next, in San Ramon, Matagalpa department, a recontra rebel was killed and another wounded in a confrontation with National Police. -
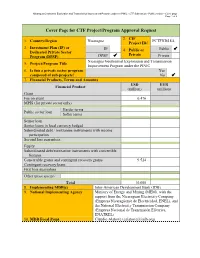
Cover Page for CTF Project/Program Approval Request
Nicaragua Geothermal Exploration and Transmission Improvement Program under the PINIC - CTF Submission - Public version – Cover page Page 1 of 6 Cover Page for CTF Project/Program Approval Request 2. CIF 1. Country/Region Nicaragua PCTFNI618A Project ID# 3. Investment Plan (IP) or IP 4. Public or Public Dedicated Private Sector Private Program (DPSP) DPSP Private Nicaragua Geothermal Exploration and Transmission 5. Project/Program Title Improvement Program under the PINIC 6. Is this a private sector program Yes composed of sub-projects? No 7. Financial Products, Terms and Amounts USD EUR Financial Product (million) (million) Grant Fee on grant 0.476 MPIS (for private sector only) Harder terms Public sector loan Softer terms Senior loan Senior loans in local currency hedged Subordinated debt / mezzanine instruments with income participation Second loss guarantees Equity Subordinated debt/mezzanine instruments with convertible features Convertible grants and contingent recovery grants 9.524 Contingent recovery loans First loss guarantees Other (please specify) Total 10.000 8. Implementing MDB(s) Inter-American Development Bank (IDB) 9. National Implementing Agency Ministry of Energy and Mining (MEM), with the support from the Nicaraguan Electricity Company (Empresa Nicaragüense de Electricidad, ENEL), and the National Electricity Transmission Company (Empresa Nacional de Transmisión Eléctrica, ENATREL) 10. MDB Focal Point Claudio Alatorre ([email protected]) Nicaragua Geothermal Exploration and Transmission Improvement Program under the PINIC - CTF Submission - Public version – Cover page Page 2 of 6 11. Brief Description of Project/Program (including objectives and expected outcomes) In 2015 electricity demand reached 665.4 MW, and it is projected to reach 896 to 1,038 MW by 2026. -

Countries at the Crossroads 2012: Nicaragua
Countries at the Crossroads COUNTRIES AT THE CROSSROADS 2012: NICARAGUA INTRODUCTION Nicaragua’s November 2011 elections marked a major step forward in President Daniel Ortega’s consolidation of power, and a served as a stark demonstration of the authoritarian tendencies he has exhibited since his return to office in early 2007. A onetime guerrilla leader and the head of the Sandinista National Liberation Front (FSLN) during the1979–1990 Sandinista revolution, Ortega has ruled Nicaragua with increasing disrespect for the constitution, electoral integrity, and the rule of law. In order to run again, he engineered a questionable ruling from the Supreme Court of Justice (CSJ) to eviscerate a constitutional ban on successive terms for sitting presidents, as well as a limitation to two total terms of office.1 Ortega then secured 62 percent of the popular vote, although irregularities were widespread enough to cast doubt on the size of his victory margin. The disputed election also gave his party, the FSLN, 63 of 92 seats in the National Assembly, a majority large enough to pass ordinary legislation, change the constitution, and even call a constitutional assembly. Armed with this supermajority, Ortega is now in a position to govern in the temperamental manner of his ideological brethren in the Bolivarian Alliance for the Americas (ALBA) headed by Venezuelan president Hugo Chávez, who has provided him an economic lifeline since 2007. During his first term as president (1984–1990), Ortega presided over the drafting of a constitution in 1987 that reflected the quasi-socialist character of the revolution, which was marked by wealth redistribution and widespread confiscations of private property. -

Nicaragua: Chronology of Political Violence & Related Events, February 4 - 10 Deborah Tyroler
University of New Mexico UNM Digital Repository NotiCen Latin America Digital Beat (LADB) 2-14-1992 Nicaragua: Chronology Of Political Violence & Related Events, February 4 - 10 Deborah Tyroler Follow this and additional works at: https://digitalrepository.unm.edu/noticen Recommended Citation Tyroler, Deborah. "Nicaragua: Chronology Of Political Violence & Related Events, February 4 - 10." (1992). https://digitalrepository.unm.edu/noticen/7071 This Article is brought to you for free and open access by the Latin America Digital Beat (LADB) at UNM Digital Repository. It has been accepted for inclusion in NotiCen by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. LADB Article Id: 062672 ISSN: 1089-1560 Nicaragua: Chronology Of Political Violence & Related Events, February 4 - 10 by Deborah Tyroler Category/Department: General Published: Friday, February 14, 1992 Feb. 4: Military spokespersons reported one recontra and one recompa killed and four other unidentified combatants wounded during a clash between recontras and recompas near Puerto Viejo, Matagalpa department. Feb. 5: Unidentified assailants burned a bus near Matiguas, Matagalpa. No report on casualties was available. Next, another unidentified group set up a roadblock near Matiguas, forcing five public transport vehicles to stop. One of the vehicles rolled over when the driver attempted to break through the roadblock. Feb. 7: An Interior Ministry spokesperson reported that 346 recontras and 70 recompas surrendered their weapons during a disarmament ceremony held at Tomatoya, Jinotega department. Recontra chiefs "Halcon" and "Culebra" were among the 346. Recontras led by commanders "Peligro," "Xavier," "Punalito," "Saltamontes," and "Terror" agreed to disarm Feb. -

Observing the 2006 Nicaragua Elections
SPECIAL REPORT SERIES Observing the 2006 Nicaragua Elections Waging Peace. Fighting Disease. Building Hope. THE CARTER CENTER STRIVES TO RELIEVE SUFFERING BY ADVANCING PEACE AND HEALTH WORLDWIDE; IT SEEKS TO PREVENT AND RESOLVE CONFLICTS, ENHANCE FREEDOM AND DEMOCRACY, AND PROTECT AND PROMOTE HUMAN RIGHTS WORLDWIDE. OBSERVING THE 2006 NICARAGUA ELECTIONS ONE COPENHILL 453 FREEDOM PARKWAY ATLANTA, GA 30307 (404) 420-5188 FAX (404) 420-5196 WWW.CARTERCENTER.ORG MAY 2007 THE CARTER CENTER OBSERVING THE 2006 NICARAGUA ELECTIONS CONTENTS Foreword . .3 Acknowledgments . .5 Mission Delegation and Staff . 8 Executive Summary . .11 The Pre-election Period . .14 Election Day and Its Aftermath . .32 Conclusions and Recommendations . .39 Appendices . .48 A. Invitation to Observe . .49 B. Role of Long-Term Observers . .51 C. Strategic Deployment of Short-Term Observers . .53 D. Representative Deployment of Short-Term Observers . .58 E. List of Short-Term Deployment Teams . .60 F. Observation Forms . .61 G. Carter Center Public Statements and Press Releases . .67 The Carter Center at a Glance . .90 THE CARTER CENTER OBSERVING THE 2006 NICARAGUA ELECTIONS FOREWORD he Carter Center has monitored national elec- tions in Nicaragua four times, beginning in 1990. Throughout the past 16 years, we have ONTEALEGRE T M sought to ensure that Nicaraguans and the internation- al community were provided with accurate information ARGARITA about the quality of the elections. Carter Center M observers and staff tracked the technical preparations and the electoral campaigns, observed the vote process and count, and provided follow-up visits to monitor electoral justice and the inauguration of new officials. We contributed our knowledge, suggesting to election authorities, political parties, and civil society ways in which the election process could be improved. -
Infected Areas As at 6 September 2001 Zones Infectées Au 6
Infected areas as at 6 September 2001 For criteria used in compiling this list, see p. 280. - Newly reported areas X Zones infectées au 6 septembre 2001 Les critères appliqués pour la compilation de cette liste, voir p. 280. - Nouvelles zones signalées X • • Bujumbura Province Ashanti Region Maputo City Province Plague Peste America Amérique Bujumbura Arrondissement Central Region Catembe District Bolivia • Bolivie Bururi Province Eastern Region Inhaça District La Paz Department Makamba Arrondissement Upper East Region Maputo Province Africa • Afrique Franz Tamayo Province Rumonge Arrondissement Volta Reg ion Boane District Sud Yungas Province Gitega Province Western Region Magude District Dem. Rep. of Congo Valle Grande Province Gitega Arrondissement Guinea • Guinée Manhica District Rép. dém. du Congo Makamba Province Maputo City Brazil • Brésil Conakry Province Haut Zaïre Province Nyanza-lac Commune Marracuene District Bahia State Forécariah Préfecture Ituri Sub-Region Cameroon • Cameroun Matola OTM District Mahagi Administrative Zone Biritinga Municipio Guinea-Bissau Moamba District Candeal Municipio Province de lExtrême-Nord Guinée-Bissau Ressano Garcia District Madagascar Central Municipio Diamare Département Sabie District Logone-et-Chari Département Bissau District Antananarivo Province Conceição Municipio Xinavane District Feira de Santana Municipio Mayo-Danai Département Biombo District Ambohidratrimo S. Préf. Gabu District Nampula Province Iraquara Municipio Mayo-Sava Département Niassa Province Antananarivo-Avaradrano S. Préf. -
Nicaragua: Government Troops Attack 500 Contras in Chontales Deborah Tyroler
University of New Mexico UNM Digital Repository NotiCen Latin America Digital Beat (LADB) 11-8-1989 Nicaragua: Government Troops Attack 500 Contras In Chontales Deborah Tyroler Follow this and additional works at: https://digitalrepository.unm.edu/noticen Recommended Citation Tyroler, Deborah. "Nicaragua: Government Troops Attack 500 Contras In Chontales." (1989). https://digitalrepository.unm.edu/ noticen/3525 This Article is brought to you for free and open access by the Latin America Digital Beat (LADB) at UNM Digital Repository. It has been accepted for inclusion in NotiCen by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. LADB Article Id: 071748 ISSN: 1089-1560 Nicaragua: Government Troops Attack 500 Contras In Chontales by Deborah Tyroler Category/Department: General Published: Wednesday, November 8, 1989 In its Nov. 3 issue, the Sandinista party newspaper Barricada reported that government troops supported by helicopter gunships attacked about 500 contras in La Pinuela and La Cusuca areas of Chontales department. Defense Ministry sources who spoke on condition of anonymity said the army was fighting contra units in the mountains that run from the northern border with Honduras to the southeast. On Nov. 2 the Defense Ministry reported that three contras were killed in El Guapote, 120 miles northeast of the capital in Matagalpa department, and that seven soldiers were wounded during combat in Pantasma and Plan de Grama, Jinotega department. On the same day, Radio Sandino reported that 400 contras who had recently infiltrated into the Quilali area and two other northern districts were "assassinating and kidnapping civilians and making propaganda for the rightist National Opposition Union coalition." (Basic data from AP, 11/03/89) -- End -- ©2011 The University of New Mexico, Latin American & Iberian Institute. -
Coffee and the Countryside: Small Farmers and Sustainable Development in Las Segovias De Nicaragua." (2012)
University of New Mexico UNM Digital Repository Anthropology ETDs Electronic Theses and Dissertations 7-1-2012 Coffee nda the Countryside: Small Farmers and Sustainable Development in Las Segovias de Nicaragua Patrick Staib Follow this and additional works at: https://digitalrepository.unm.edu/anth_etds Part of the Anthropology Commons Recommended Citation Staib, Patrick. "Coffee and the Countryside: Small Farmers and Sustainable Development in Las Segovias de Nicaragua." (2012). https://digitalrepository.unm.edu/anth_etds/66 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Anthropology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Patrick W. Staib Candidate Anthropology Department This dissertation is approved, and it is acceptable in quality and form for publication: Approved by the Dissertation Committee: Dr. Les W. Field , Chairperson Dr. Carole Nagengast Dr. Marta Weigle Dr. David Henkel i COFFEE AND THE COUNTRYSIDE: SMALL FARMERS AND SUSTAINABLE DEVELOPMENT IN LAS SEGOVIAS DE NICARAGUA by PATRICK W. STAIB B.A., Spanish and Religion, Dickinson College, 1995 M.A., Latin American studies, University of New Mexico, 2000 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Anthropology The University of New Mexico Albuquerque, New Mexico July, 2012 ii COFFEE AND THE COUNTRYSIDE: SMALL FARMERS AND SUSTAINABLE DEVELOPMENT IN LAS SEGOVIAS DE NICARAGUA by Patrick W. Staib B.A., DICKINSON COLLEGE, 1995 M.A., UNIVERSITY OF NEW MEXICO, 2000 Ph.D., UNIVERSITY OF NEW MEXICO, 2012 ABSTRACT Las Segovias de Nicaragua is a highland tropical region along the Río Coco and the Honduran border in the northern part of the country.