Biofluorescence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M7 Electroluminescence of Polymers
Universität Potsdam Institute of Physics and Astronomy Advanced Physics Lab Course March 2020 M7 ELECTROLUMINESCENCE OF POLYMERS I. INTRODUCTION The recombination of holes and electrons in a luminescent material can produce light. This emitted light is referred to as electroluminescence (EL) and was discovered in organic single crystals by Pope, Magnante, and Kallmann in 1963.[1] EL from conjugated polymers was first reported by Burroughes et al.[2] The polymer used was poly(p-phenylenevinylene) (PPV). Since then, a variety of other polymers has been investigated. Organic EL devices have applications in a wide field ranging from multi-color displays and optical information processing to lighting. Polymers have the advantage over inorganic and monomolecular materials in the ease with which thin, structurally robust and large area films can be perpared from solutions. Using printing techniques, patterned structures can be produced easily. Even flexible displays can be produced because of the good mechanical properties of polymers. In this lab course, basic optical and electrical properties of conjugated polymers will be investigated. Advanced Lab Course: Electroluminescence of Polymers 2 EXPERIMENTAL TASKS Measure the absorption spectra of your polymers (thin films spin coated onto glass substrates). Characterize the setup used for luminescence measurements. Identify possible sources of error and collect data necessary for their correction. Measure the photoluminescence emission spectra for the polymer films, using suitable excitation wavelengths. Measure the photoluminescence excitation spectra for the polymer films, using suitable detection wavelengths. Measure the current through the OLEDs and the spectral radiant intensity of electroluminescence as a function of applied voltage (the current-radiance-voltage characteristics). -

Article Soot Photometer Using Supervised Machine Learning
Atmos. Meas. Tech., 12, 3885–3906, 2019 https://doi.org/10.5194/amt-12-3885-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Classification of iron oxide aerosols by a single particle soot photometer using supervised machine learning Kara D. Lamb1,2 1Cooperative Institute for Research in Environmental Sciences, University of Colorado Boulder, Boulder, CO, USA 2NOAA Earth System Research Laboratory Chemical Sciences Division, Boulder, CO, USA Correspondence: Kara D. Lamb ([email protected]) Received: 15 March 2019 – Discussion started: 22 March 2019 Revised: 20 June 2019 – Accepted: 21 June 2019 – Published: 15 July 2019 Abstract. Single particle soot photometers (SP2) use laser- each class are compared with the true class for those particles induced incandescence to detect aerosols on a single particle to estimate generalization performance. While the specific basis. SP2s that have been modified to provide greater spec- class approach performed well for rBC and Fe3O4 (≥ 99 % tral contrast between their narrow and broad-band incandes- of these aerosols are correctly identified), its classification of cent detectors have previously been used to characterize both other aerosol types is significantly worse (only 47 %–66 % refractory black carbon (rBC) and light-absorbing metallic of other particles are correctly identified). Using the broader aerosols, including iron oxides (FeOx). However, single par- class approach, we find a classification accuracy of 99 % for ticles cannot be unambiguously identified from their incan- FeOx samples measured in the laboratory. The method al- descent peak height (a function of particle mass) and color lows for classification of FeOx as anthropogenic or dust-like ratio (a measure of blackbody temperature) alone. -

Chemiluminescence Western Blotting Technical Guide and Protocols
TECH TIP Chemiluminescent substrates Chemiluminescence western blotting technical guide and protocols Introduction Types and considerations of chemiluminescence Western blotting is a powerful and commonly used tool western blotting to identify and quantify a specific protein in a complex HRP is the most popular enzyme used in western mixture. As originally conceived by Towbin et al., the blotting and will be discussed throughout this document technique enables indirect detection of protein samples as our example. The most suitable western blotting immobilized on a nitrocellulose or polyvinylidene fluoride substrates for HRP are luminol-based, and they produce (PVDF) membrane. In a conventional western blot, a chemiluminescent signal. Chemiluminescence is a protein samples are first resolved by sodium dodecyl chemical reaction that produces energy released in the sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) form of light. In the presence of HRP and a peroxide buffer, and then electrophoretically transferred to the membrane. luminol oxidizes and forms an excited-state product that Following a blocking step, the membrane is probed emits light as it decays to the ground state. Light emission with a primary antibody (polyclonal or monoclonal) occurs only during the enzyme–substrate reaction and, that was raised against the antigen in question. After a therefore, once the substrate in proximity to the enzyme is subsequent washing step, the membrane is incubated with exhausted, signal output ceases. In contrast, colorimetric a secondary antibody conjugated to an enzyme that is substrates, such as diaminobenzidine (DAB), produce reactive toward the primary antibody. An enzyme such as precipitate that remains visible on the membrane even alkaline phosphatase (AP) or horseradish peroxidase (HRP) after the reaction has terminated. -

Introduction 1
1 1 Introduction . ex arte calcinati, et illuminato aeri [ . properly calcinated, and illuminated seu solis radiis, seu fl ammae either by sunlight or fl ames, they conceive fulgoribus expositi, lucem inde sine light from themselves without heat; . ] calore concipiunt in sese; . Licetus, 1640 (about the Bologna stone) 1.1 What Is Luminescence? The word luminescence, which comes from the Latin (lumen = light) was fi rst introduced as luminescenz by the physicist and science historian Eilhardt Wiede- mann in 1888, to describe “ all those phenomena of light which are not solely conditioned by the rise in temperature,” as opposed to incandescence. Lumines- cence is often considered as cold light whereas incandescence is hot light. Luminescence is more precisely defi ned as follows: spontaneous emission of radia- tion from an electronically excited species or from a vibrationally excited species not in thermal equilibrium with its environment. 1) The various types of lumines- cence are classifi ed according to the mode of excitation (see Table 1.1 ). Luminescent compounds can be of very different kinds: • Organic compounds : aromatic hydrocarbons (naphthalene, anthracene, phenan- threne, pyrene, perylene, porphyrins, phtalocyanins, etc.) and derivatives, dyes (fl uorescein, rhodamines, coumarins, oxazines), polyenes, diphenylpolyenes, some amino acids (tryptophan, tyrosine, phenylalanine), etc. + 3 + 3 + • Inorganic compounds : uranyl ion (UO 2 ), lanthanide ions (e.g., Eu , Tb ), doped glasses (e.g., with Nd, Mn, Ce, Sn, Cu, Ag), crystals (ZnS, CdS, ZnSe, CdSe, 3 + GaS, GaP, Al 2 O3 /Cr (ruby)), semiconductor nanocrystals (e.g., CdSe), metal clusters, carbon nanotubes and some fullerenes, etc. 1) Braslavsky , S. et al . ( 2007 ) Glossary of terms used in photochemistry , Pure Appl. -

New Lighting—New Leds
New Lighting—New LEDs Aspects on light‐emitting diodes from social and material science perspectives Editors Mats Bladh & Mikael Syväjärvi Published by Linköping University Electronic Press, 2010 ISBN: 978‐91‐7393‐270‐7 URL: http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva‐60807 © The Authors Contents Foreword ...................................................................................... 5 Authors ........................................................................................ 7 Introduction: A Paradigmatic Shift? Mats Bladh & Mikael Syväjärvi ................................................................................. 9 Materials and Growth Technologies for Efficient LEDs Mikael Syväjärvi, Satoshi Kamiyama, Rositza Yakimova & Isamu Akasaki ............... 16 Light Excitation and Extraction in LEDs Satoshi Kamiyama, Motoaki Iwaya, Isamu Akasaki, Mikael Syväjärvi & Rositza Yakimova ...................................................................................................... 27 ‘No Blue’ White LED Haiyan Ou, Dennis Corell, Carsten Dam‐Hansen, Paul‐Michael Petersen & Dan Friis .................................................................................................................... 35 User Responses to Energy Efficient Light Sources in Home Environments Monica Säter ............................................................................................................. 43 Prospects for LED from a Historical Perspective Mats Bladh ............................................................................................................... -
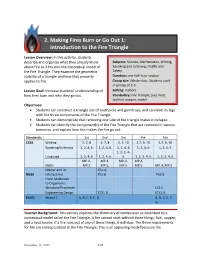
2. Making Fires Burn Or Go out 1: Introduction to the Fire Triangle
2. Making Fires Burn or Go Out 1: Introduction to the Fire Triangle Lesson Overview: In this activity, students describe and organize what they already know Subjects: Science, Mathematics, Writing, about fire so it fits into the conceptual model of Speaking and Listening, Health and the Fire Triangle. They examine the geometric Safety stability of a triangle and how that property Duration: one half-hour session applies to fire. Group size: Whole class. Students work in groups of 2-3. Lesson Goal: Increase students’ understanding of Setting: Indoors how fires burn and why they go out. Vocabulary: Fire Triangle, fuel, heat, ignition, oxygen, model Objectives: • Students can construct a triangle out of toothpicks and gumdrops, and can label its legs with the three components of the Fire Triangle. • Students can demonstrate that removing one side of the triangle makes it collapse. • Students can identify the component(s) of the Fire Triangle that are removed in various scenarios, and explain how this makes the fire go out. Standards: 1st 2nd 3rd 4th 5th CCSS Writing 2, 7, 8 2, 7, 8 2, 7, 10 2,7, 9, 10 2,7, 9, 10 Speaking/Listening 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 3, 4, Language 1, 2, 4, 6 1, 2, 4, 6 6 1, 2, 3, 4, 6 1, 2, 3, 4, 6 MP.4, MP.4, MP.4, MP.4, Math MP.5 MP.5, MP.5 MP.5 MP.4, MP.5 Matter and Its PS1.A, NGSS Interactions PS1.B PS1.B From Molecules to Organisms: Structure/Processes LS1.C Engineering Design ETS1.B ETS1.B EEEGL Strand 1 A, B, C, E, F, G A, B, C, E, F, G Teacher Background: This activity explores the chemistry of combustion as described by a conceptual model called the Fire Triangle. -

Energy Efficiency Lighting Hearing Committee On
S. HRG. 110–195 ENERGY EFFICIENCY LIGHTING HEARING BEFORE THE COMMITTEE ON ENERGY AND NATURAL RESOURCES UNITED STATES SENATE ONE HUNDRED TENTH CONGRESS FIRST SESSION TO RECEIVE TESTIMONY ON THE STATUS OF ENERGY EFFICIENT LIGHT- ING TECHNOLOGIES AND ON S. 2017, THE ENERGY EFFICIENT LIGHT- ING FOR A BRIGHTER TOMORROW ACT SEPTEMBER 12, 2007 ( Printed for the use of the Committee on Energy and Natural Resources U.S. GOVERNMENT PRINTING OFFICE 39–385 PDF WASHINGTON : 2007 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate 0ct 09 2002 11:58 Feb 20, 2008 Jkt 040443 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 G:\DOCS\39385.XXX SENERGY2 PsN: MONICA COMMITTEE ON ENERGY AND NATURAL RESOURCES JEFF BINGAMAN, New Mexico, Chairman DANIEL K. AKAKA, Hawaii PETE V. DOMENICI, New Mexico BYRON L. DORGAN, North Dakota LARRY E. CRAIG, Idaho RON WYDEN, Oregon LISA MURKOWSKI, Alaska TIM JOHNSON, South Dakota RICHARD BURR, North Carolina MARY L. LANDRIEU, Louisiana JIM DEMINT, South Carolina MARIA CANTWELL, Washington BOB CORKER, Tennessee KEN SALAZAR, Colorado JOHN BARRASSO, Wyoming ROBERT MENENDEZ, New Jersey JEFF SESSIONS, Alabama BLANCHE L. LINCOLN, Arkansas GORDON H. SMITH, Oregon BERNARD SANDERS, Vermont JIM BUNNING, Kentucky JON TESTER, Montana MEL MARTINEZ, Florida ROBERT M. SIMON, Staff Director SAM E. FOWLER, Chief Counsel FRANK MACCHIAROLA, Republican Staff Director JUDITH K. PENSABENE, Republican Chief Counsel (II) VerDate 0ct 09 2002 11:58 Feb 20, 2008 Jkt 040443 PO 00000 Frm 00002 Fmt 5904 Sfmt 5904 G:\DOCS\39385.XXX SENERGY2 PsN: MONICA C O N T E N T S STATEMENTS Page Bingaman, Hon. -

Lecture ˮincoherent Light Sources“ Summer Term 2008 Tim Pohle Electroluminescence Light Sources – Table of Contents
Lecture ˮIncoherent Light Sources“ Summer term 2008 Tim Pohle Electroluminescence Light Sources – Table of contents Table of contents . Overview of electroluminescence . LED (Light Emitting Diode) History of development Technical details Applications . OLED (Organic Light Emitting Diode) History of development Technical details Applications . Electroluminescent strings and foils – Light Emitting Capacitor History of development Technical details Applications Incoherent Light Sources 2008 Tim Pohle 2 Electroluminescence Light Sources – Overview of Electroluminescence Overview of Electroluminescence [6] [3] . It depeds on luminescence [1] . It is distinct from fluorescence3/phosphorescence, [2] chemoluminescence2, sonoluminescence5, bioluminescence1, superluminescence6, triboluminescnece4, etc. [4] [5] . It is an optical/electrical phenomenon . Material emits light in response to an electric current passed through it, or to a strong electric field CB . It is result of radiative recombination of electrons and holes in a material VB Incoherent Light Sources 2008 Tim Pohle 3 Electroluminescence Light Sources – LED LED (Light Emitting Diode) History of development 1907 Henry Joseph Round (Marconi Labs) „invents the LED“. He finds out that some inorganic substances glow if a electric voltage is impress on them. He publishted his observation in the journal „Electrical World“ 1921 Ignorant of Round`s invention, Oleg Vladimirovich Losev makes the same observations 1927- Losev investigates this effect 1942 and guesses that it is the inversion of Einstein„s photoelectrical effect Incoherent Light Sources 2008 Tim Pohle 4 Electroluminescence Light Sources – LED 1951 Satisfactory explanation of the light emission due to the semiconductor and transistor development 1961 Bob Biard and Gary Pittman (Texas Instruments) find out that gallium arsenide (GaAs) give off infrared radiation when electric current is applied. -

Chem 257 Lecture for Exp. 8, 2004 1. Exp. 7 Report: to Be Handed in This Week, Along With: • Relevant Notebook Pages • Vial
Chem 257 Lecture for Exp. 8, 2004 1. Exp. 7 Report: To be handed in this week, along with: • Relevant notebook pages • Vial containing triphenyl carbinol labeled with your notebook code, i.e., JST-I-38a 2. Exp. 8 Quiz. Be prepared for Quiz. 3. Required Reading. Lab Manual 8.1-10. 4. Relevant Material in Wade (5th): Ultraviolet spectroscopy 15-13 (p 666-672), 16-15 p(711-713), 18-5E (p 785-6). Friedel Crafts Alkylation: 17-11 (p 746-748) & p 980. Exp. 8. Fluorescence and Chemiluminescence. Purpose of the experiment: 1. Synthetic Organic Chemistry • Acquaint you with Friedel-Crafts acylation chemistry 2. Physical Organic Chemistry • Introduce you to photophysics • Acquaint you with chemiluminescent reactions 1 Photochemical Terms and Principles. Useful to consider a ground and excited state energy diagram for an organic molecule. Energy Level Diagram ISC S = singlet S T = triplet 1 A = Absorption F = Fluorescence T IC 1 P = Phosphorescence A ISC = Intersystem crossing E F P IC = Internal Conversion ISC Absorption of a photon Emmission of a photon Vibrational energy loss So Singlet State (S) • Spin paired. • Ground state usually a singlet (So). • First excited singlet state = S1 So S1 • Nth excited singlet state = S n ground excited Triplet State (T) • Spin unpaired. • Lowest triplet state is usually an excited state (T1). • Nth excited triplet state = Tn T T Exceptions: 0 1 • Oxygen is a ground state Ground excited triplet! (O2) 2 Selection rules. • Spin allowed transitions do not involve a change in spin state (Sn → Sm, Tn → Tm). • Thus ground singlet states are most easily excited to their first excited singlet states (So → S1). -

Properties of Light Library Document
Light + Air North America Properties of Light Library Document 2 Properties Library Document of Light Properties of Light Light as energy The other way of representing light is as a wave phenomenon. This is somewhat more difficult for most people to understand, but Light is remarkable. It is something we take for granted every day, perhaps an analogy with sound waves will be useful. When you play but it is not something we stop and think about very often or even a high note and a low note on the piano, they both produce sound, try to define. Let us take a few minutes and try to understand but the main thing that is different between the two notes is the some things about light. Simply stated, light is nature’s way frequency of the vibrating string producing the sound waves--the of transferring energy through space. We can complicate it by faster the vibration the higher the pitch of the note. If we now shift talking about interacting electric and magnetic fields, quantum our focus to the sound waves themselves instead of the vibrating mechanics and all of that, but just remember, light is energy. Light string, we would find that the higher pitched notes have shorter travels very rapidly, but it does have a finite velocity. In vacuum, wavelengths, or distances between each successive wave. Likewise the speed of light is 186,282 miles per second (or nearly 300,000 (and restricting ourselves to optical light for the moment), blue kilometers per second), which is really humming along! However, light and red light are both just light, but the blue light has a higher when we start talking about the incredible distances in astronomy, frequency of vibration (or a shorter wavelength) than the red light. -

Measuring Peroxyoxalate Chemiluminescence Using a Spectrofluorophotometer No.A493
LAAN-A-RF-E003 Application Spectrophotometric Analysis News Measuring Peroxyoxalate Chemiluminescence Using a Spectrofluorophotometer No.A493 Chemiluminescence is a phenomenon where molecules n Fluorescent Dyes Used in Glow Sticks are excited by a chemical reaction and then emit light Commercially marketed glow sticks are shown in Fig. 2. energy as they return to ground state. Chemiluminescence The oxalate ester and fluorescent dye solution are placed based on using oxalate esters features high-emission in a sealed thin-walled glass container and the glass efficiency and long emission time and provides container is placed inside a polyethylene tube together illumination for long periods without any electricity. with hydrogen peroxide solution to which a catalyst is Consequently, it is used for recreational, fishing, and many added. Bending the polyethylene tube breaks the glass other applications where it is commonly called glow sticks. container, which causes both solutions to mix together The following describes the luminescent process of and emit light. peroxyoxalate chemiluminescence and gives an example Examples of the fluorescent dyes used in glow sticks are of using an RF-6000 spectrofluorophotometer to measure shown in Fig. 3. Polycyclic aromatic fluorescent dyes are the emission spectra of glow sticks. used, which emit different colors based on the wavelength of light emitted when the fluorescent dyes change from excited to ground state. n Luminescent Process of Peroxyoxalate Chemiluminescence Peroxyoxalate chemiluminescence is caused by a chemical O RO O reaction between an oxalate ester and hydrogen peroxide O C within a fluorescent dye solution. As shown in Fig. 1, the C C + H2O2 2ROH + O C oxalate ester is oxidized by hydrogen peroxide to produce O OR O ROH and 1,2-dioxetanedione. -

Top- and Bottom-Emission-Enhanced Electroluminescence of Deep-UV
OPEN Top- and bottom-emission-enhanced SUBJECT AREAS: electroluminescence of deep-UV INORGANIC LEDS NANOPHOTONICS AND light-emitting diodes induced by PLASMONICS localised surface plasmons Received 26 November 2013 Kai Huang, Na Gao, Chunzi Wang, Xue Chen, Jinchai Li, Shuping Li, Xu Yang & Junyong Kang Accepted 27 February 2014 Department of Physics, Fujian Provincial Key Laboratory of Semiconductor Materials and Application, Xiamen University, Xiamen, 361005, P. R. China. Published 14 March 2014 We report localised-surface-plasmon (LSP) enhanced deep-ultraviolet light-emitting diodes (deep-UV LEDs) using Al nanoparticles for LSP coupling. Polygonal Al nanoparticles were fabricated on the top surfaces of the deep-UV LEDs using the oblique-angle deposition method. Both the top- and Correspondence and bottom-emission electroluminescence of deep-UV LEDs with 279 nm multiple-quantum-well emissions requests for materials can be effectively enhanced by the coupling with the LSP generated in the Al nanoparticles. The primary should be addressed to bottom-emission wavelength is longer than the primary top-emission wavelength. This difference in K.H. (k_huang@xmu. wavelength can be attributed to the substrate-induced Fano resonance effect. For resonance modes with edu.cn) or J.C.L. shorter wavelengths, the radiation fraction directed back into the LEDs is largest in the direction that is nearly parallel to the surface of the device and results in total reflection and re-absorption in the LEDs. ([email protected]) emiconductor deep-ultraviolet (deep-UV) light sources based on III-nitride light-emitting diodes (LEDs) have been intensively investigated because of their potential applications, including air and water purifica- tion, germicidal and biomedical instrumentation systems, and ophthalmic surgery tools1–3.