Basic Physics of the Incandescent Lamp (Lightbulb) Dan Macisaac, Gary Kanner,Andgraydon Anderson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

16 CFR Ch. I (1–1–20 Edition) § 305.22
Federal Trade Commission Pt. 305 PART 305—ENERGY AND WATER 305.27 Paper catalogs and websites. USE LABELING FOR CONSUMER ADDITIONAL REQUIREMENTS PRODUCTS UNDER THE ENERGY 305.28 Test data records. POLICY AND CONSERVATION 305.29 Required testing by designated lab- ACT (‘‘ENERGY LABELING oratory. RULE’’) EFFECT OF THIS PART SCOPE 305.30 Effect on other law. 305.31 Stayed or invalid parts. Sec. 305.32 [Reserved] 305.1 Scope of the regulations in this part. APPENDIX A1 TO PART 305—REFRIGERATORS DEFINITIONS WITH AUTOMATIC DEFROST APPENDIX A2 TO PART 305—REFRIGERATORS 305.2 Definitions. AND REFRIGERATOR-FREEZERS WITH MAN- 305.3 Description of appliances and con- UAL DEFROST sumer electronics. APPENDIX A3 TO PART 305—REFRIGERATOR- 305.4 Description of furnaces and central air FREEZERS WITH PARTIAL AUTOMATIC DE- conditioners. FROST 305.5 Description of lighting products. APPENDIX A4 TO PART 305—REFRIGERATOR- 305.6 Description of plumbing products. FREEZERS WITH AUTOMATIC DEFROST GENERAL WITH TOP-MOUNTED FREEZER NO THROUGH-THE-DOOR ICE 305.7 Prohibited acts. APPENDIX A5 TO PART 305—REFRIGERATOR- FREEZERS WITH AUTOMATED DEFROST TESTING WITH SIDE-MOUNTED FREEZER NO 305.8 Determinations of estimated annual THROUGH-THE-DOOR ICE energy consumption, estimated annual APPENDIX A6 TO PART 305—REFRIGERATOR- operating cost, and energy efficiency rat- FREEZERS WITH AUTOMATED DEFROST ing, water use rate, and other required WITH BOTTOM-MOUNTED FREEZER NO disclosure content. THROUGH-THE-DOOR ICE 305.9 Duty to provide labels on websites. APPENDIX A7 TO PART 305—REFRIGERATOR- 305.10 Determinations of capacity. FREEZERS WITH AUTOMATIC DEFROST 305.11 Submission of data. -
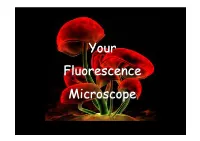
Your Fluorescence Microscope Transmitted-Light
Your Fluorescence Microscope Transmitted-light. Bright-field Bright-field microscopy = Transmitted-light INVERTED UPRIGHT Fluorescence microscopy = Reflected-light Mercury Lamp Heat Filter Emission Filter Mirror Excitation Filter Collimating Lens Dichromatic Mirror (From:http://micro.magnet.fsu.edu) You need to know … Your light source Your filters Your objective Your detector Spectrum of a Mercury Lamp Your Light Source • Mercury lamp Wavelength (nm) • Xenon lamp Spectrum of a Xenon Lamp • Metal halide lamp • Halogen lamp • LED • Laser Wavelength (nm) (Modified from: h6p://www.cairn-research.co.uk) Your Light Source 1) Halogen lamp 2) Mercury lamp 3) Xenon lamp 4) Metal halide lamp 5) LED 6) Laser Tungsten – Halogen lamp • White light source • Inexpensive long lasNng bulbs • Used mainly for brighQield illuminaNon • CAN be used for fluorescence excitaNon above 400nm • Ideal for live cell imaging (low power, no UV) • “Colour” changes with temperature Mercury (HBO) lamp PROS • white light source • 10-100x brighter then halogen • focused intensity light-source • very bright intensity peaks at specific wavelengths for many standard fluoreophores CONS • short bulb life (≈200-400h) • generates a lot of heat • requires bulb alignment • no uniform intensity (peaks) • bulb are hazardous waste • long warm-up time • excitation wavelength cannot be • Intensity decay over Nme, intensity controlled independently flickering Xenon lamp PROS • white light source • relaNvely even intensity across visible spectrum • focused intense light source CONS • requires bulb alignment • bulbs are hazardous waste • Intensity decay over Nme • weaker intensity in UV • generates a lot of heat in the IR region • relaNvely low power in visible range • excitaNon wavelength cannot be controlled independently Metal Halide lamp PROS • white light source • brighter intensity between peaks than mercury lamp • no bulb alignment, more uniform field of illum. -

Article Soot Photometer Using Supervised Machine Learning
Atmos. Meas. Tech., 12, 3885–3906, 2019 https://doi.org/10.5194/amt-12-3885-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Classification of iron oxide aerosols by a single particle soot photometer using supervised machine learning Kara D. Lamb1,2 1Cooperative Institute for Research in Environmental Sciences, University of Colorado Boulder, Boulder, CO, USA 2NOAA Earth System Research Laboratory Chemical Sciences Division, Boulder, CO, USA Correspondence: Kara D. Lamb ([email protected]) Received: 15 March 2019 – Discussion started: 22 March 2019 Revised: 20 June 2019 – Accepted: 21 June 2019 – Published: 15 July 2019 Abstract. Single particle soot photometers (SP2) use laser- each class are compared with the true class for those particles induced incandescence to detect aerosols on a single particle to estimate generalization performance. While the specific basis. SP2s that have been modified to provide greater spec- class approach performed well for rBC and Fe3O4 (≥ 99 % tral contrast between their narrow and broad-band incandes- of these aerosols are correctly identified), its classification of cent detectors have previously been used to characterize both other aerosol types is significantly worse (only 47 %–66 % refractory black carbon (rBC) and light-absorbing metallic of other particles are correctly identified). Using the broader aerosols, including iron oxides (FeOx). However, single par- class approach, we find a classification accuracy of 99 % for ticles cannot be unambiguously identified from their incan- FeOx samples measured in the laboratory. The method al- descent peak height (a function of particle mass) and color lows for classification of FeOx as anthropogenic or dust-like ratio (a measure of blackbody temperature) alone. -

Introduction 1
1 1 Introduction . ex arte calcinati, et illuminato aeri [ . properly calcinated, and illuminated seu solis radiis, seu fl ammae either by sunlight or fl ames, they conceive fulgoribus expositi, lucem inde sine light from themselves without heat; . ] calore concipiunt in sese; . Licetus, 1640 (about the Bologna stone) 1.1 What Is Luminescence? The word luminescence, which comes from the Latin (lumen = light) was fi rst introduced as luminescenz by the physicist and science historian Eilhardt Wiede- mann in 1888, to describe “ all those phenomena of light which are not solely conditioned by the rise in temperature,” as opposed to incandescence. Lumines- cence is often considered as cold light whereas incandescence is hot light. Luminescence is more precisely defi ned as follows: spontaneous emission of radia- tion from an electronically excited species or from a vibrationally excited species not in thermal equilibrium with its environment. 1) The various types of lumines- cence are classifi ed according to the mode of excitation (see Table 1.1 ). Luminescent compounds can be of very different kinds: • Organic compounds : aromatic hydrocarbons (naphthalene, anthracene, phenan- threne, pyrene, perylene, porphyrins, phtalocyanins, etc.) and derivatives, dyes (fl uorescein, rhodamines, coumarins, oxazines), polyenes, diphenylpolyenes, some amino acids (tryptophan, tyrosine, phenylalanine), etc. + 3 + 3 + • Inorganic compounds : uranyl ion (UO 2 ), lanthanide ions (e.g., Eu , Tb ), doped glasses (e.g., with Nd, Mn, Ce, Sn, Cu, Ag), crystals (ZnS, CdS, ZnSe, CdSe, 3 + GaS, GaP, Al 2 O3 /Cr (ruby)), semiconductor nanocrystals (e.g., CdSe), metal clusters, carbon nanotubes and some fullerenes, etc. 1) Braslavsky , S. et al . ( 2007 ) Glossary of terms used in photochemistry , Pure Appl. -

Indoor Lighting Design Guide
CSU Office of the Chancellor Indoor Lighting Design Guide Indoor Lighting Design Guide Rev: 12/12/18 CSU Office of the Chancellor Indoor Lighting Design Guide ACKNOWLEDGEMENT The California State University (CSU) gratefully acknowledges the effort and work of Jai Agaram, John Andary, Douglas Effenberger, Kent Peterson, Steven Strauss, and Steve Taylor. Comments or inquiries may be directed to: The California State University Office of the Chancellor Capital Planning Design and Construction Long Beach, California Attention: Thomas Kennedy, Chief Architecture and Engineering Telephone: (562) 951-4129 E-mail: [email protected] CSU Office of the Chancellor Indoor Lighting Design Guide TABLE OF CONTENTS EXECUTIVE SUMMARY .......................................................................................................................................................... 1 SECTION 1: INTRODUCTION ............................................................................................................................................ 2 CSU POLICY .................................................................................................................................................................................. 2 DEFINITIONS .................................................................................................................................................................................. 2 UTILITY INCENTIVES ....................................................................................................................................................................... -

Effects of Sio2/Al2o3 Ratios on Sintering Characteristics of Synthetic Coal Ash
Article Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash Hongwei Hu 1, Kun Zhou 1, Kesheng Meng 1,2, Lanbo Song 1 and Qizhao Lin 1,* 1 Department of Thermal Science and Energy Engineering, University of Science and Technology of China, Hefei 230027, China; [email protected] (H.H.); [email protected] (K.Z.); [email protected] (K.M.); [email protected] (L.S.) 2 Anhui Civil Aviation Airport Group, Hefei 230086, China * Correspondence: [email protected]; Tel.: +86-551-6360-0430; Fax: +86-551-6360-3487 Academic Editor: Mehrdad Massoudi Received: 01 December 2016; Accepted: 14 February 2017; Published: 16 February 2017 Abstract: This article explores the effects of SiO2/Al2O3 ratios (S/A) on sintering characteristics and provides guidance for alleviating ash depositions in a large-scale circulation fluidized bed. Five synthetic coal ash (SCA) samples with different S/As were treated in a muffle furnace for 12 h at different temperatures (from 773 K to 1373 K, in 100 K intervals). The morphological and chemical results of the volume shrinkage ratio (VSR), thermal deformation analysis by dilatometer (DIL), scanning electron microscope (SEM), X-ray photoelectron spectrometer (XPS), and X-ray diffraction (XRD) were combined to describe the sintering characteristics of different samples. The results showed that the sintering procedure mainly occurred in the third sintering stage when the temperature was over 1273 K, accompanied with significant decreases in the VSR curve. Excess SiO2 (S/A = 4.5) resulted in a porous structure while excess Al2O3 (S/A = 0.5) brought out large aggregations. -

Industrial Lighting a Primer
Industrial Lighting A Primer All through history people have sought better ways to illuminate their work. Even the cavemen needed torches to allow them to draw on the walls of their underground caverns. Fire brought both light and heat for thousands of years before crude lamps of animal fat gave way to the candle for general indoor illumination used around the world. Roman Grease Lamp An offshoot of the common candlestick became what we now call the Lacemakers Globe. This quite possibly could qualify as the world’s first industrial light source! It was observed that when a candle flame was aligned behind a rounded glass bottle filled with water, a magnifying and focusing effect was produced, in addition to simply lighting up a small area. This was high technology of the first order! No longer did the lacemaker have to pack it in when the sun went down, for soon the new Lacemaker’s Globe became fairly common in European Cottage Industry, enabling the production of lace at a greater rate than ever before. It is also not too much of a stretch to conclude that the repetitive patterns of the lacemaker could be looked upon as a forerunner to the theory of mass production, along with the pin makers who toiled away in the ‘second tier’ of the feeble light pool cast by the globe, hammering heads onto straightened bits of wire in order to make the common pin. This was mighty slim pickings by today’s standards to be sure, but just a few hundred years ago it represented a revolutionary increase in handiwork production after the sun went down, for the very first time in history. -

Fluorescence Cell Imaging and Manipulation Using Conventional Halogen Lamp Microscopy
Fluorescence Cell Imaging and Manipulation Using Conventional Halogen Lamp Microscopy Kazuo Yamagata1,2, Daisaku Iwamoto3, Yukari Terashita1,4, Chong Li1, Sayaka Wakayama1,Yoko Hayashi-Takanaka5, Hiroshi Kimura5, Kazuhiro Saeki3, Teruhiko Wakayama1* 1 RIKEN Center for Developmental Biology, Kobe, Japan, 2 Research Institute for Microbial Research, Osaka University, Suita, Japan, 3 Department of Genetic Engineering, Kinki University, Kinokawa, Wakayama, Japan, 4 Laboratory of Animal Reproduction, Graduate School of Agricultural Science, Tohoku University, Sendai, Japan, 5 Graduate School of Frontier Biosciences, Osaka University, Suita, Japan Abstract Technologies for vitally labeling cells with fluorescent dyes have advanced remarkably. However, to excite fluorescent dyes currently requires powerful illumination, which can cause phototoxic damage to the cells and increases the cost of microscopy. We have developed a filter system to excite fluorescent dyes using a conventional transmission microscope equipped with a halogen lamp. This method allows us to observe previously invisible cell organelles, such as the metaphase spindle of oocytes, without causing phototoxicity. Cells remain healthy even after intensive manipulation under fluorescence observation, such as during bovine, porcine and mouse somatic cell cloning using nuclear transfer. This method does not require expensive epifluorescence equipment and so could help to reduce the science gap between developed and developing countries. Citation: Yamagata K, Iwamoto D, Terashita Y, Li C, Wakayama S, et al. (2012) Fluorescence Cell Imaging and Manipulation Using Conventional Halogen Lamp Microscopy. PLoS ONE 7(2): e31638. doi:10.1371/journal.pone.0031638 Editor: Sue Cotterill, St. Georges University of London, United Kingdom Received September 12, 2011; Accepted January 10, 2012; Published February 8, 2012 Copyright: ß 2012 Yamagata et al. -
2. Making Fires Burn Or Go out 1: Introduction to the Fire Triangle
2. Making Fires Burn or Go Out 1: Introduction to the Fire Triangle Lesson Overview: In this activity, students describe and organize what they already know Subjects: Science, Mathematics, Writing, about fire so it fits into the conceptual model of Speaking and Listening, Health and the Fire Triangle. They examine the geometric Safety stability of a triangle and how that property Duration: one half-hour session applies to fire. Group size: Whole class. Students work in groups of 2-3. Lesson Goal: Increase students’ understanding of Setting: Indoors how fires burn and why they go out. Vocabulary: Fire Triangle, fuel, heat, ignition, oxygen, model Objectives: • Students can construct a triangle out of toothpicks and gumdrops, and can label its legs with the three components of the Fire Triangle. • Students can demonstrate that removing one side of the triangle makes it collapse. • Students can identify the component(s) of the Fire Triangle that are removed in various scenarios, and explain how this makes the fire go out. Standards: 1st 2nd 3rd 4th 5th CCSS Writing 2, 7, 8 2, 7, 8 2, 7, 10 2,7, 9, 10 2,7, 9, 10 Speaking/Listening 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 4, 6 1, 2, 3, 4, Language 1, 2, 4, 6 1, 2, 4, 6 6 1, 2, 3, 4, 6 1, 2, 3, 4, 6 MP.4, MP.4, MP.4, MP.4, Math MP.5 MP.5, MP.5 MP.5 MP.4, MP.5 Matter and Its PS1.A, NGSS Interactions PS1.B PS1.B From Molecules to Organisms: Structure/Processes LS1.C Engineering Design ETS1.B ETS1.B EEEGL Strand 1 A, B, C, E, F, G A, B, C, E, F, G Teacher Background: This activity explores the chemistry of combustion as described by a conceptual model called the Fire Triangle. -

Energy Efficiency Lighting Hearing Committee On
S. HRG. 110–195 ENERGY EFFICIENCY LIGHTING HEARING BEFORE THE COMMITTEE ON ENERGY AND NATURAL RESOURCES UNITED STATES SENATE ONE HUNDRED TENTH CONGRESS FIRST SESSION TO RECEIVE TESTIMONY ON THE STATUS OF ENERGY EFFICIENT LIGHT- ING TECHNOLOGIES AND ON S. 2017, THE ENERGY EFFICIENT LIGHT- ING FOR A BRIGHTER TOMORROW ACT SEPTEMBER 12, 2007 ( Printed for the use of the Committee on Energy and Natural Resources U.S. GOVERNMENT PRINTING OFFICE 39–385 PDF WASHINGTON : 2007 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate 0ct 09 2002 11:58 Feb 20, 2008 Jkt 040443 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 G:\DOCS\39385.XXX SENERGY2 PsN: MONICA COMMITTEE ON ENERGY AND NATURAL RESOURCES JEFF BINGAMAN, New Mexico, Chairman DANIEL K. AKAKA, Hawaii PETE V. DOMENICI, New Mexico BYRON L. DORGAN, North Dakota LARRY E. CRAIG, Idaho RON WYDEN, Oregon LISA MURKOWSKI, Alaska TIM JOHNSON, South Dakota RICHARD BURR, North Carolina MARY L. LANDRIEU, Louisiana JIM DEMINT, South Carolina MARIA CANTWELL, Washington BOB CORKER, Tennessee KEN SALAZAR, Colorado JOHN BARRASSO, Wyoming ROBERT MENENDEZ, New Jersey JEFF SESSIONS, Alabama BLANCHE L. LINCOLN, Arkansas GORDON H. SMITH, Oregon BERNARD SANDERS, Vermont JIM BUNNING, Kentucky JON TESTER, Montana MEL MARTINEZ, Florida ROBERT M. SIMON, Staff Director SAM E. FOWLER, Chief Counsel FRANK MACCHIAROLA, Republican Staff Director JUDITH K. PENSABENE, Republican Chief Counsel (II) VerDate 0ct 09 2002 11:58 Feb 20, 2008 Jkt 040443 PO 00000 Frm 00002 Fmt 5904 Sfmt 5904 G:\DOCS\39385.XXX SENERGY2 PsN: MONICA C O N T E N T S STATEMENTS Page Bingaman, Hon. -

Nanoparticle Sintering
Application Note Nanoparticle Sintering Introduction the catalyst to be studied, and (iii) is observed (Fig. 2a). Analysis of Δλ preventing the catalyst from directly during the course of the experiment, Catalyst sintering is a major cause of interacting with the Au nanodisks by shows that the LSPR shifts fast in catalyst deactivation, which yearly e.g. alloy formation. the beginning and then more slowly causes billions of dollars of extra cost The Pt model catalyst is formed onto towards the end of the experiment associated with catalyst regeneration the sensor chip by evaporating a 0.5 (Fig. 2b, black curve). In contrast, and renewal. nm thick (nominal thickness) granu- when the same experiment is per- In order to develop more sintering- lar film. This results in individual Pt formed in pure Ar (or in 4% O2 on resistant catalysts, a detailed under- nanoparticles with an average diam- a “blank” sensor without Pt) no standing of the sintering kinetics and eter of <D> = 3.3 nm (+/- 1.1 nm), significant shift during the entire ex- mechanisms is required. It is therefore which mimics the size range of real periment is observed (Fig. 2b, blue of great importance to investigate sin- supported catalysts. curve). These results indicate that tering in situ, in real time and under The change of the localized surface INPS can be used to monitor the realistic catalyst operation conditions plasmon resonance (LSPR) centroid sintering of nanoparticle catalysts (i.e. at high temperatures and pres- wavelength, Δλ, is monitored during (since O2 is a known sintering pro- sures in reactive gas atmospheres). -

!History of Lightingv2.Qxd
CONTENTS Introduction 3 The role of lighting in modern society 3 1. The oldest light sources 4 Before the advent of the lamp 4 The oldest lamps 4 Candles and torches 5 Further development of the oil lamp 6 2. Gaslight 9 Introduction 9 Early history 9 Gas production 10 Gaslight burners 10 The gas mantle 11 3. Electric lighting before the incandescent lamp 14 Introduction 14 Principle of the arc lamp 15 Further development of the arc lamp 16 Applications of the arc lamp 17 4. The incandescent lamp 20 The forerunners 20 The birth of the carbon-filament lamp 22 Further development of the carbon-filament lamp 25 Early metal-filament lamps 27 The Nernst lamp 28 The birth of the tungsten-filament lamp 29 Drawn tungsten filaments 30 Coiled filaments 30 The halogen incandescent lamp 31 5. Discharge lamps 32 Introduction 32 The beginning 32 High-voltage lamps 33 Early low-pressure mercury lamps 34 The fluorescent lamp 35 High-pressure mercury lamps 36 Sodium lamps 37 The xenon lamp 38 6. Electricity production and distribution 39 Introduction 39 Influence machines and batteries 39 Magneto-electric generators 40 Self-exciting generators 41 The oldest public electricity supply systems 41 The battle of systems 42 The advent of modern a.c. networks 43 The History of Light and Lighting While the lighting industry is generally recognized as being born in 1879 with the introduction of Thomas Alva Edison’s incandescent light bulb, the real story of light begins thousands of years earlier. This brochure was developed to provide an extensive look at one of the most important inventions in mankind’s history: artificial lighting.