!History of Lightingv2.Qxd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Electric Arc Plasma Temperature
DIAGNOSTICS OF MULTICOMPONENT ELECTRIC ARC PLASMA A.I. Cheredarchuk, V.F. Boretskij, A.N. Veklich Taras Shevchenko Kiev National University, Kiev, Ukraine E-mail: [email protected] The plasma parameters of electric arc discharge in a gas flow between copper electrodes were investigated. The electrical conductivity of copper-argon and copper-carbon dioxide plasma was calculated. It was found that at low temperatures (5000 K<T<9000 K) the electrical conductivity considerably depends on the amount of copper in plasma. PACS: 51.20.d 1. INTRODUCTION Ne(r) in discharge at 30 A were obtained from width of The electric arc between evaporated electrodes has spectral lines CuI 448.0 nm, broadened due to the quadratic diverse technological applications. It is well known that Stark effect. In the case of low current (3.5 A) absolute electrode vapours have a determining influence on intensity of CuI 465.1 nm spectral line was used. properties of arc plasma. The insignificant impurity N , m-3 (about 1 %) of electrode metal vapour appreciably j CO 2 changes plasma parameters of the discharge in a rather CO wide temperature range [1]. Unfortunately the influence O O e of metal impurity on the plasma of free burning electric 1E23 2 C arc discharge in different working gases is not O+ experimentally investigated in detail yet. The main aim of this study is a development of complex diagnostics techniques of determination of 1E20 plasma parameters of electric arc discharge in a gas flow between copper electrodes. + CO 2. TRANSPORT PROPERTIES C 2 T, K 1E17 OF THERMAL PLASMA 5000 10000 15000 20000 In calculations of the transport properties of thermal plasma it is necessary to know the proportion between Fig. -

Industrial Lighting a Primer
Industrial Lighting A Primer All through history people have sought better ways to illuminate their work. Even the cavemen needed torches to allow them to draw on the walls of their underground caverns. Fire brought both light and heat for thousands of years before crude lamps of animal fat gave way to the candle for general indoor illumination used around the world. Roman Grease Lamp An offshoot of the common candlestick became what we now call the Lacemakers Globe. This quite possibly could qualify as the world’s first industrial light source! It was observed that when a candle flame was aligned behind a rounded glass bottle filled with water, a magnifying and focusing effect was produced, in addition to simply lighting up a small area. This was high technology of the first order! No longer did the lacemaker have to pack it in when the sun went down, for soon the new Lacemaker’s Globe became fairly common in European Cottage Industry, enabling the production of lace at a greater rate than ever before. It is also not too much of a stretch to conclude that the repetitive patterns of the lacemaker could be looked upon as a forerunner to the theory of mass production, along with the pin makers who toiled away in the ‘second tier’ of the feeble light pool cast by the globe, hammering heads onto straightened bits of wire in order to make the common pin. This was mighty slim pickings by today’s standards to be sure, but just a few hundred years ago it represented a revolutionary increase in handiwork production after the sun went down, for the very first time in history. -

Selective Radiation from Various Substances, Iii
. SELECTIVE RADIATION FROM VARIOUS SUBSTANCES, III By W. W. Coblentz CONTENTS Page. I. Introduction 243 II. Apparatus and Methods 244 1 The Spectrometer 244 2. Comparison of Dispersion of Prisms 246 3. The Radiometers 247 4. The Rotating Sectored Disk 249 5. Accuracy Attainable 252 III. The Acetylene Flame.' 253 1 Historical 253 2. The Visible Spectrum 257 3. The Emissivity a Function of the Thickness of the Radiating Layer. 261 4. Comparison of Acetylene Flame and a Solid Radiator 264 5. Absorptivity of an Acetylene Flame 266 6. Summary 273 IV. The Selective Emission oif the Welsbach Mantle 274 1 Historical 274 2. The Visible Spectrum 277 3. Comparison of a Gas Mantle with a Solid Glower 278 V. Miscellaneous Substances 287 1 Emission of Arc of Nemst Glower Materials 287 2. Color Match Versus Spectral Intensity Match 288 I. INTRODUCTION The present investigation is the beginning of an attempt to supply a long-felt need of an accurate knov/ledge of the spectral energy distribution, of some standard sources of Hght, in the visible and in the ultra-violet parts of the spectrum. The problem is a difficult one and heretofore has never been given thorough attention. Once the spectral energy distribution of a standard source of illumination is accurately known, it is possible to obtain the spectral energy distribution of other weaker sources of radia- tion by indirect methods, such as by spectrophotography or spectrophotometry. The standard sources which are the most easily reproduced are probably well seasoned incandescent lamps 243 244 Bulletin of the Bureau of Standards [Voi. -

Electric Light Pdf, Epub, Ebook
ELECTRIC LIGHT PDF, EPUB, EBOOK Seamus Heaney | 96 pages | 19 Mar 2001 | FABER & FABER | 9780571207985 | English | London, United Kingdom Electric Light PDF Book Tour EL: 5 min videos on each light type, followed by a 5 question quiz for each lamp type. Wednesday 17 June In , Thomas Edison began serious research into developing a practical incandescent lamp and on October 14, , Edison filed his first patent application for "Improvement In Electric Lights". Sunday 27 September Wednesday 29 July Wednesday 16 September View all albums. Saturday 10 October View all similar artists. In colder climates where heating and lighting is required during the cold and dark winter months, the heat byproduct has some value. Saturday 15 August Similar To Jeff Lynne. Tuesday 18 August Monday 25 May Wednesday 22 April Play album Buy Loading. Friday 24 April Wednesday 15 July Main article: Incandescent light bulb. Wednesday 14 October Help Learn to edit Community portal Recent changes Upload file. Features Exploring the local sounds and scenes at Noise Pop Fest. Due to the importance of this area of engineering we offer a full course of web pages, videos, and educational tools to communicate to you the world of of the electric light and the engineers and inventors who made it possible. Friday 31 July Monday 8 June Friday 21 August Sunday 18 October Friday 26 June The inside of the tubes are coated with phosphors that give off visible light when struck by ultraviolet photons. Friday 4 September Connect to Spotify Dismiss. Thursday 7 May Sunday 31 May Wednesday 1 July Tuesday 20 October The electric arc is struck by touching the rod tips then separating them. -
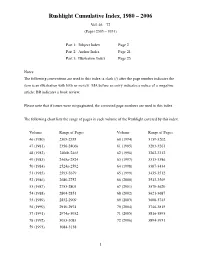
Rushlight Index 1980-2006
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004 -

The Electric-Lamp Industry
Massachusetts Institute of Technology Studies of Innovation • GiSma,..=("EaEssormat THE MACMILLAN COMPANY THE ELECTRIC-LAMP INDUSTRY: NEW YORK a BOSTON a CHICAGO DALLAS • ATLANTA • SAN FRANCISCO MACMILLAN AND CO., LIMITED Technological Change and Economic LONDON a BOMBAY a CALCUTTA MADRAS a MELBOURNE Development from 1800 to 1947 THE MACMILLAN COMPANY OF CANADA, LIMITED TORONTO By ARTHUR A. BRIGHT, Jr. THE MACMILLAN COMPANY • NEW YORK 1949 FOREWORD THIS study of the economic development of the electric- lamp industry is the second volume in a series of studies on the economics of innovation, undertaken at the Massachusetts Insti- tute of Technology. The creative role played by science and technology in modern economic life is apparent to everyone. But we know relatively little about the human factors which condition the introduction of technological change into our environment. Are there barriers to innovation inherent in the increasing concentration of power in a few large concerns? Does the patent system, designed as an incentive to invention, act more often as a brake on new develop- ments? What has been the role of key personalities in creating change? Are there lessons to be drawn from the past on how the innovating process can be more effective, not only from the standpoint of achieving a higher standard of material being but from the point of view of smoother human relations? Certainly, material progress at any price is not a satisfactory goal. On the other hand, freedom for creative action in initiating and carrying out new developments is a basic human drive for many individu- als. I believe, personally, that a great society should strive toward a goal which will give to individuals and groups the maximum opportunities for creative expression; yet this means to me that the State must act to prevent the compulsive pressure of some particular group from overriding others to the destruction of human values. -

Humphry Davy and the Arc Light
REMAKING HISTORY By William Gurstelle Humphry Davy and the Arc Light » Thomas Edison did not invent the first electric BRILLIANT light.* More than 70 years before Edison’s 1879 MISTAKES: Humphry Davy, incandescent lamp patent, the English scientist chemist, inventor, Humphry Davy developed a technique for produc- and philosopher: ing controlled light from electricity. “I have learned Sir Humphry Davy (1778–1829) was one of the more from my failures than from giants of 19th-century science. A fellow of the my successes.” prestigious Royal Society, Davy is credited with discovering, and first isolating, elemental sodium, potassium, calcium, magnesium, boron, barium, and strontium. A pioneer in electrochemistry, he appeared between the electrode tips, Davy had to also developed the first medical use of nitrous oxide separate the carbon electrodes slightly and care- and invented the miner’s safety lamp. The safety fully in order to sustain the continuous, bright arc lamp alone is directly responsible for saving of electricity. Once that was accomplished, he found hundreds, if not thousands, of miners’ lives. the device could sustain the arc for long periods, But it is his invention of the arc lamp for which we even as the carbon rods were consumed in the heat remember him here. Davy’s artificial electric light of the process. consisted of two carbon rods, made from wood Davy’s arc lamp of 1807 was not economically charcoal, connected to the terminals of an enormous practical until the cost of producing a 50V-or-so collection of voltaic cells. (In Davy’s day, thousands power supply became reasonable. -

High Performance Flash and Arc Lamps Catalog
Europe: Saxon Way, Bar Hill, Cambridge, CB3 8SL Tel: +44 (0)1954 782266 Fax: +44 (0)1954 782993 USA: 44370 Christy St., Freemont, CA 94538, USA Tel: (800) 775-OPTO Tel: (510) 979-6500 Fax: (510) 687-1344 USA: 35 Congress Street, Salem, MA 01970, USA Tel: (978) 745-3200 Fax: (978) 745-0894 Asia: 47 Ayer Rajah Cresent, 06-12, Singapore 139947 Tel: +65-775-2022 Fax: +65-775-1008 Japan: 18F, Parale Mitsui Building 8, Higashida-cho, Kawasaki-ku, Kawasaki-shi, Kanagawa-ken, 210-0005 Japan Tel: 81 44 200 9150 Fax: 81 44 200 9160 www.perkinelmer.com/opto Optoelectronics Lighting Imaging Telecom High Performance Flash and Arc Lamps Lighting Imaging Teleco m Introduction This publication is divided into two sections: Past, Present and Future Part 1 – Technical Information Solid state laser systems have historically used pulsed and CW (DC) xenon or krypton filled arc lamps as exci- Part 2 – Product Range tation for pump sources. When in 1960, at Hughes Research Labs, the first practical pulsed laser system Part 1 is intended to give the necessary technical infor- was demonstrated by mation to manufacturers, designers and researchers to T. H. Maiman the technology and understanding enable them to select the correct flashlamp for their involved in the manufacture and operation of application and also to give an insight into the design flashlamps was of a very basic nature. Up to procedures necessary for correct flashlamp operation. that time (1960) the major use of flashlamps was pho- Part 2 is a guide to the wide, varied and complex range tography and related applications. -

History of Electric Light
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 76. NUMBER 2 HISTORY OF ELECTRIC LIGHT BY HENRY SGHROEDER Harrison, New Jersey PER\ ^"^^3^ /ORB (Publication 2717) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION AUGUST 15, 1923 Zrtie Boxb QSaftitnore (prcee BALTIMORE, MD., U. S. A. CONTENTS PAGE List of Illustrations v Foreword ix Chronology of Electric Light xi Early Records of Electricity and Magnetism i Machines Generating Electricity by Friction 2 The Leyden Jar 3 Electricity Generated by Chemical Means 3 Improvement of Volta's Battery 5 Davy's Discoveries 5 Researches of Oersted, Ampere, Schweigger and Sturgeon 6 Ohm's Law 7 Invention of the Dynamo 7 Daniell's Battery 10 Grove's Battery 11 Grove's Demonstration of Incandescent Lighting 12 Grenet Battery 13 De Moleyns' Incandescent Lamp 13 Early Developments of the Arc Lamp 14 Joule's Law 16 Starr's Incandescent Lamp 17 Other Early Incandescent Lamps 19 Further Arc Lamp Developments 20 Development of the Dynamo, 1840-1860 24 The First Commercial Installation of an Electric Light 25 Further Dynamo Developments 27 Russian Incandescent Lamp Inventors 30 The Jablochkofif " Candle " 31 Commercial Introduction of the Differentially Controlled Arc Lamp ^3 Arc Lighting in the United States 3;^ Other American Arc Light Systems 40 " Sub-Dividing the Electric Light " 42 Edison's Invention of a Practical Incandescent Lamp 43 Edison's Three-Wire System 53 Development of the Alternating Current Constant Potential System 54 Incandescent Lamp Developments, 1884-1894 56 The Edison " Municipal -

Zallen Gsas.Harvard 0084L 11460.Pdf (8.379Mb)
American Lucifers: Makers and Masters of the Means of Light, 1750-1900 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Zallen, Jeremy Benjamin. 2014. American Lucifers: Makers and Masters of the Means of Light, 1750-1900. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12274111 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA American Lucifers: Makers and Masters of the Means of Light, 1750-1900 A dissertation presented by Jeremy Benjamin Zallen to The Department of History in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of History Harvard University Cambridge, Massachusetts April 2014 ©2014—Jeremy Benjamin Zallen All rights reserved. American Lucifers: Makers and Masters of the Means of Light, 1750-1900 Abstract This dissertation examines the social history of Atlantic and American free and unfree labor by focusing on the production and consumption of the means of light from the colonial period to the end of the nineteenth century. Drawing from archives across the country, I reconstruct the ground-level experiences and struggles of the living (and dying) bringers of lights—those American lucifers—and the worlds they made in the process. I begin by arguing that colonial American deep-sea whaling voyages triggered an Atlantic street lighting revolution radiating from London, while a New England run candles-for-slave(ry) trade helped illuminate and circulate processes caught up in colonial transatlantic sugar slavery. -

Ann Cooper Hewitt G.S
HIGH SOCIETY The Curious Case of Ann Cooper Hewitt G.S. Payne looks at a long-forgotten scandal that rocked America’s high society in the 1930s “I’M ONLY A sterilized heiress, foundries, as well as from a litany nia millionaire who came from A butt for the laughter of rubes, of inventions that ranged from the one of the oldest, wealthiest fami- I’m comely and rich, first US steam locomotive to the lies in New York society. Where But a venomous bitch — first gelatin dessert (later to exactly Maryon came from My mother ran off with my tubes!” become better known by its brand remains sketchy. She seemed to — Gene Fowler, journalist/humorist, name, Jell-O). Cooper’s son-in- have cultivated an image of a pop- the New York Daily Mirror, 1936 law, Abram Stevens Hewitt, was a ular Southern belle from Virginia. It was the story of the year, mayor of New York City and gen- But according to daughter Ann, the sensational scandal everyone erally regarded as the father of the “While my mother has always was talking about from New York New York City subway system. boasted of her Southern aristoc- to San Francisco. Ann Cooper Cooper’s grandson (Ann’s father) racy, she was the daughter of a Hewitt was the heiress to a horsecar driver in San Francisco fortune, and she was suing who lived in a flat over a corner her own mother, Maryon grocery store when she was a Cooper Hewitt, for half a mil- girl.” lion dollars. Why? Because Wherever she came from, two years earlier, in 1934, Maryon found her way into when Ann was 20 and still money and society with her technically a minor, Maryon marriage to Brugiere. -

The Invention of the Electric Light
The Invention of the Electric Light B. J. G. van der Kooij This case study is part of the research work in preparation for a doctorate-dissertation to be obtained from the University of Technology, Delft, The Netherlands (www.tudelft.nl). It is one of a series of case studies about “Innovation” under the title “The Invention Series”. About the text—This is a scholarly case study describing the historic developments that resulted in the steam engine. It is based on a large number of historic and contemporary sources. As we did not conduct any research into primary sources, we made use of the efforts of numerous others by citing them quite extensively to preserve the original character of their contributions. Where possible we identified the individual authors of the citations. As some are not identifiable, we identified the source of the text. Facts that are considered to be of a general character in the public domain are not cited. About the pictures—Many of the pictures used in this case study were found at websites accessed through the Internet. Where possible they were traced to their origins, which, when found, were indicated as the source. As most are out of copyright, we feel that the fair use we make of the pictures to illustrate the scholarly case is not an infringement of copyright. Copyright © 2015 B. J. G. van der Kooij Cover art is a line drawing of Edison’s incandescent lamp (US Patent № 223.898) and Jablochkoff’s arc lamp (US Patent № 190.864) (courtesy USPTO). Version 1.1 (April 2015) All rights reserved.