Leleux Press Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
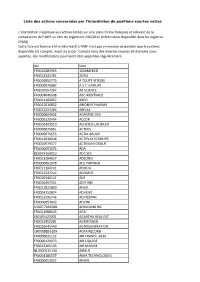
Liste Des Actions Concernées Par L'interdiction De Positions Courtes Nettes
Liste des actions concernées par l'interdiction de positions courtes nettes L’interdiction s’applique aux actions listées sur une plate-forme française et relevant de la compétence de l’AMF au titre du règlement 236/2012 (information disponible dans les registres ESMA). Cette liste est fournie à titre informatif. L'AMF n'est pas en mesure de garantir que le contenu disponible est complet, exact ou à jour. Compte tenu des diverses sources de données sous- jacentes, des modifications pourraient être apportées régulièrement. Isin Nom FR0010285965 1000MERCIS FR0013341781 2CRSI FR0010050773 A TOUTE VITESSE FR0000076887 A.S.T. GROUPE FR0010557264 AB SCIENCE FR0004040608 ABC ARBITRAGE FR0013185857 ABEO FR0012616852 ABIONYX PHARMA FR0012333284 ABIVAX FR0000064602 ACANTHE DEV. FR0000120404 ACCOR FR0010493510 ACHETER-LOUER.FR FR0000076861 ACTEOS FR0000076655 ACTIA GROUP FR0011038348 ACTIPLAY (GROUPE) FR0010979377 ACTIVIUM GROUP FR0000053076 ADA BE0974269012 ADC SIIC FR0013284627 ADEUNIS FR0000062978 ADL PARTNER FR0011184241 ADOCIA FR0013247244 ADOMOS FR0010340141 ADP FR0010457531 ADTHINK FR0012821890 ADUX FR0004152874 ADVENIS FR0013296746 ADVICENNE FR0000053043 ADVINI US00774B2088 AERKOMM INC FR0011908045 AG3I ES0105422002 AGARTHA REAL EST FR0013452281 AGRIPOWER FR0010641449 AGROGENERATION CH0008853209 AGTA RECORD FR0000031122 AIR FRANCE -KLM FR0000120073 AIR LIQUIDE FR0013285103 AIR MARINE NL0000235190 AIRBUS FR0004180537 AKKA TECHNOLOGIES FR0000053027 AKWEL FR0000060402 ALBIOMA FR0013258662 ALD FR0000054652 ALES GROUPE FR0000053324 ALPES (COMPAGNIE) -

Document De Référence 2014 Document De Référence
DOCUMENT DE RÉFÉRENCE 2014 DBV TECHNOLOGIES 2014 DOCUMENT DE RÉFÉRENCE DOCUMENT 2014 DE RÉFÉRENCE DBV TECHNOLOGIES Société Anonyme au capital de 1 966 166,10 euros Green Square Bâtiment D, 80/84 rue des Meuniers 92220 Bagneux 441 772 522 R.C.S. Nanterre En application de son règlement général, notamment de l’article 212-13, l’Autorité des marchés financiers a enregistré le présent document de référence le 02 juillet 2015 sous le numéro R.15-057. Ce document ne peut être utilisé à l’appui d’une opération financière que s’il est complété par une note d’opération visée par l’AMF. Il a été établi par l’émetteur et engage la responsabilité de ses signataires. L’enregistrement, conformément aux dispositions de l’article L. 621-8-1-I du code monétaire et financier, a été effectué après que l’AMF a vérifié que le document est complet et compréhensible et que les informations qu’il contient sont cohérentes. Il n’implique pas l’authentification par l’AMF des éléments comptables et financiers présentés. Incorporation par référence : En application de l’article 28 du règlement européen 809/2004, les éléments suivants sont inclus par référence dans le présent document de référence : • Les comptes annuels établis conformément aux principes comptables français au 31 décembre 2013, les comptes établis selon le référentiel IFRS tel qu’adoptés dans l’Union européenne au 31 décembre 2013, et les rapports des commissaires aux comptes y afférents, présentés respectivement aux pages 153 à 190, 191 à 208, 209 et 210-211 du documents de référence n° R.14-017 enregistré par l’Autorités des marchés financiers le 16 avril 2014 ; • Les comptes annuels établis conformément aux principes comptables français au 31 décembre 2012, les comptes établis selon le référentiel IFRS tel qu’adoptés dans l’Union européenne au 31 décembre 2012, et les rapports des commissaires aux comptes y afférents, présentés respectivement aux pages 134 à 162, 163 à 178, 180, 181-182 du document de référence n° R.13-015 enregistré par l’Autorité des marchés financiers le 24 avril 2013. -

Sony Corporation Announces Signing of a Definitive Agreement for the Acquisition of Equity Interest in Insomniac Games, Inc
August 19, 2019 Sony Corporation Sony Corporation Announces Signing of a Definitive Agreement for the Acquisition of Equity Interest in Insomniac Games, Inc. by its Wholly-owned Subsidiary August 19, 2019 (Pacific Standard Time) – Sony Interactive Entertainment LLC (“SIE”), a wholly-owned subsidiary of Sony Corporation (“Sony”), today announced that SIE has entered into definitive agreements to acquire the entire equity interest in Insomniac Games, Inc. Completion of the acquisition is subject to regulatory approvals and certain other closing conditions. Financial terms of this transaction are not disclosed due to contractual commitments. For further details, please refer to the attached press release. This transaction is not anticipated to have a material impact on Sony's forecast for its consolidated financial results for the fiscal year ending March 31, 2020. SONY INTERACTIVE ENTERTAINMENT TO ACQUIRE INSOMNIAC GAMES, DEVELOPER OF PLAYSTATION®4 TOP-SELLING MARVEL’S SPIDER-MAN, RATCHET & CLANK ~Highly-acclaimed Game Creators to Join Sony Interactive Entertainment’s Worldwide Studios to Deliver Extraordinary Gaming Experiences~ SAN MATEO, Calif., August 19, 2019 - Sony Interactive Entertainment (“SIE”) announced today that SIE has entered into definitive agreements to acquire Insomniac Games, Inc. (“Insomniac Games”), a leading game developer and long-time partner of SIE, in its entirety. Insomniac Games is the developer of PlayStation®4’s (PS4™) top-selling Marvel’s Spider-Man and the hugely popular PlayStation® Ratchet & Clank franchise. Upon completion of the acquisition, Insomniac Games will join the global development operation of Sony Interactive Entertainment Worldwide Studios (“SIE WWS”). Insomniac Games is the 14th studio to join the SIE WWS family. -

Putnam Panagora Market Neutral Fund Q3 Portfolio Holdings
Putnam PanAgora Market Neutral Fund The fund's portfolio 5/31/20 (Unaudited) INVESTMENT COMPANIES (46.1%)(a) Shares Value Morgan Stanley Emerging Markets Domestic Debt Fund, Inc. 640 $3,635 State Street Institutional U.S. Government Money Market Fund 3,939,067 3,939,067 Total investment companies (cost $3,943,561) $3,942,702 UNITS (11.0%)(a) Units Value Acamar Partners Acquisition Corp.(NON) 419 $4,291 Alussa Energy Acquisition Corp. (Cayman Islands)(NON) 856 8,483 Amplitude Healthcare Acquisition Corp.(NON) 2,947 29,529 B. Riley Principal Merger Corp. II(NON) 2,620 26,174 CC Neuberger Principal Holdings I(NON) 2,652 27,024 Chardan Healthcare Acquisition 2 Corp.(NON) 2,652 26,493 CHP Merger Corp.(NON) 2,747 27,745 CIIG Merger Corp.(NON) 4,529 45,335 Collective Growth Corp.(NON) 2,803 27,890 DFP Healthcare Acquisitions Corp.(NON) 2,866 28,746 dMY Technology Group, Inc.(NON) 2,885 29,196 East Stone Acquisition Corp.(NON) 4,230 42,089 FinServ Acquisition Corp.(NON) 831 8,194 Foley Trasimene Acquisition Corp.(NON) 2,626 26,917 Fortress Value Acquisition Corp.(NON) 2,652 26,547 Galileo Acquisition Corp.(NON) 888 8,827 GigCapital3, Inc.(NON) 2,833 28,160 Gores Holdings IV, Inc.(NON) 1,306 13,844 Greenrose Acquisition Corp.(NON) 3,350 32,931 GX Acquisition Corp.(NON) 417 4,233 Healthcare Merger Corp.(NON) 2,705 28,105 InterPrivate Acquisition Corp.(NON) 2,918 29,180 Jaws Acquisition Corp.(NON) 2,620 27,038 Juniper Industrial Holdings, Inc.(NON) 841 8,418 Landcadia Holdings II, Inc.(NON) 1,165 12,174 LGL Systems Acquisition Corp.(NON) 2,568 25,629 Lifesci Acquisition Corp.(NON) 2,866 29,806 LIV Capital Acquisition Corp. -

ESMA Report on Application of AMP 2020
Report To the European Commission on the application of accepted market practices 16 December 2020 | ESMA70-156-3782 ESMA REGULAR USE Table of Contents 1 Executive Summary ....................................................................................................... 2 2 Background .................................................................................................................... 3 3 Information on the legal situation of AMPs established under MAD and AMPs established under MAR ............................................................................................................................ 5 3.1 CNMV ..................................................................................................................... 5 3.2 CMVM ..................................................................................................................... 6 3.3 CONSOB ................................................................................................................ 6 3.4 AMF ........................................................................................................................ 6 4 Application of the established AMPs............................................................................... 7 4.1 AMP established by the CNMV under MAR ............................................................ 7 4.2 AMP established by the CMVM under MAR ............................................................ 8 4.3 AMP established by CONSOB under MAD and MAR ............................................. -

MEDIA RELEASE for Immediate Release APPROVED INVESTMENTS in the MANUFACTURING, SERVICES and PRIMARY SECTORS in 2019 TOTALLED
MEDIA RELEASE For Immediate Release APPROVED INVESTMENTS IN THE MANUFACTURING, SERVICES AND PRIMARY SECTORS IN 2019 TOTALLED RM207.9 BILLION Kuala Lumpur, 20 April 2020 – Malaysia has been proactive during the Movement Control Order (MCO) in balancing public health and the livelihood of the people as well as strengthening the economic fundamentals by providing the necessary approval for companies in several economic sectors to operate, and are subject to strict adherence to health and safety guidelines. “While the COVID-19 pandemic has changed the global industrial system, MITI is committed to ensuring that Malaysia continues to be positioned as an investor-friendly location for long term growth of both foreign and domestic businesses. Foreign direct investment (FDI) is a long term capital flow. We trust that the existing foreign companies will continue to weather the storm and retain their investment in the country,” said YB Dato’ Seri Mohamed Azmin Ali, Senior Minister and Minister of International Trade and Industry (MITI), today. Against the backdrop of a challenging external environment and declining global FDI inflows, Malaysia remains resilient and attracted a total of RM207.9 billion of approved investments in the manufacturing, services and primary sectors in 2019, a 1.7 per cent increase, compared to 2018. With a contribution of 60.4 per cent (RM125.5 billion), domestic direct investment (DDI) accounted for the bulk of the total approved investments. Although FDI made up for 39.6 per cent (RM82.4 billion) of the total, the value of FDI in 2019 had increased by 2.9 per cent from the previous year. -

Finding Aid to the Insomniac Games Records, 1998-2005
Brian Sutton-Smith Library and Archives of Play Insomniac Games Records Finding Aid to the Insomniac Games Records, 1998-2005 Summary Information Title: Insomniac Games records Creator: Insomniac Games, Inc. (primary) ID: 119.6337 Date: 1998-2005 (inclusive) Extent: 2.1 linear feet Language: The materials in this collection are in English. Abstract: The Insomniac Games records are a compilation of game design documentation, including notes, descriptions, drawings, and more, for various games created by Insomniac Games. The materials are dated between 1998 and 2005. Repository: Brian Sutton-Smith Library and Archives of Play at The Strong One Manhattan Square Rochester, New York 14607 585.263.2700 [email protected] Administrative Information Conditions Governing Use: This collection is open for research use by staff of The Strong and by users of its library and archives. Though the donor has not transferred intellectual property rights (including, but not limited to any copyright, trademark, and associated rights therein) to The Strong, he has given permission for The Strong to make copies in all media for museum, educational, and research purposes. Intellectual property rights for this collection are owned and controlled by Insomniac Games, Sony Interactive Entertainment, and Activision. Custodial History: The Insomniac Games records were donated to The Strong in November 2019 as a gift of John Fiorito, Insomniac Games. The papers were accessioned by The Strong under Object ID 119.6337 and were received from Fiorito in 2 boxes. Preferred citation for publication: Insomniac Games records, Brian Sutton-Smith Library and Archives of Play at The Strong Processed by: Julia Novakovic, February 2020 Controlled Access Terms Personal Names • Chew, Jacinda • Fiorito, John • Hastings, Alex • Hastings, Brian • Price, Ted Corporate Names • Activision (Firm) • Insomniac Games, Inc. -

Structures De Gouvernance Des Sociétés Cotées Radiographie
Structures de gouvernance des sociétés cotées Radiographie Observatoire Capital humain Centre de Gouvernement d'Entreprise Octobre 2015 Second line optional lorem ipsum B Subhead lorem ipsum, date quatueriure Mesurer pour comprendre, comprendre pour agir, agir pour progresser Sommaire Executive Summary 2 Préambule 4 1. Radiographie des conseils 7 Introduction 7 Typologie des conseils 8 Indépendance des administrateurs 12 Féminisation des conseils 14 Internationalisation des conseils 18 Distribution par âge et par ancienneté 20 Cumul des mandats 22 Mercato 2014-2015 25 Rémunérations des administrateurs 29 Evaluation des conseils 38 2. Les comités du Conseil 42 Introduction 43 Comités spécialisés 45 3. Assemblées générales 2015 52 Introduction 53 Nature des résolutions 54 Résultats des votes 56 Facteurs d'influence 66 Liste des sociétés 70 Structures de gouvernance des sociétés cotées - Radiographie 1 Executive Summary Des pratiques globalement en ligne avec les codes de gouvernance, mais des différences marquées selon l'indice de cotation Nous sommes très heureux de vous présenter la Nous étudierons les comités spécialisés, leur fréquence radiographie de la gouvernance des sociétés cotées et leur composition, ainsi que la distribution des rôles 2015, issue de l’analyse des pratiques de gouvernement des administrateurs en leur sein. d’entreprise de plus de 300 sociétés cotées au marché Le chapitre relatif aux assemblées générales 2015 Euronext de la Bourse de Paris. analysera près de 5 700 résolutions présentées lors Le gouvernement d’entreprise désigne les modes de 318 assemblées générales en rapprochant les taux de fonctionnement des organes d'administration et d’approbation, la nature des résolutions, la structure de surveillance d’une organisation et leurs relations actionnariale des sociétés et les recommandations de avec la direction et les actionnaires. -

France FTT List of Eligible Companies
ISIN Code Security Name Effective Date FR0000120404 ACCOR August 1, 2012 US00435F3091 ACCOR SPONSORED ADR March 2, 2015 FR0010340141 AEROPORTS DE PARIS August 1, 2012 US0091191082 AIR FRANCE - KLM ADR SPONSORED December 1, 2012 FR0000031122 AIR FRANCE-KLM August 1, 2012 FR0000120073 AIR LIQUIDE August 1, 2012 US0091262024 AIR LIQUIDE ADR December 1, 2012 FR0000130007 ALCATEL LUCENT August 1, 2012 US0212442075 ALSTOM ADR December 1, 2012 FR0010220475 ALSTOM REGROUPT August 1, 2012 FR0000033219 ALTAREA August 1, 2012 FR0000071946 ALTEN January 1, 2014 US02209U1088 ALTRAN TECHN.ADR SPONSORED January 1, 2014 FR0000034639 ALTRAN TECHNOLOGIES January 1, 2014 FR0004125920 AMUNDI January 1, 2016 FR0011027143 AREVA August 1, 2012 US04012G1022 AREVA ADR UNSPONSORED December 1, 2012 FR0010313833 ARKEMA August 1, 2012 US0412321095 ARKEMA ADR December 1, 2012 FR0000076952 ARTOIS (INDLE ET FIN.) NOM. January 1, 2014 FR0000051732 ATOS August 1, 2012 US04962A1051 ATOS ADR UNSPONSORED December 1, 2012 FR0000120628 AXA August 1, 2012 US0545361075 AXA UAP ADR SPONSORED December 1, 2012 FR0000035164 BENETEAU January 1, 2016 FR0000120966 BIC August 1, 2012 US0887361030 BIC ADR December 1, 2012 FR0010096479 BIOMERIEUX August 1, 2012 US09074E1010 BIOMERIEUX ADR September 17, 2015 FR0000131104 BNP PARIBAS ACTIONS A August 1, 2012 US05565A1034 BNP PARIBAS ADR SPONSORED December 1, 2012 US05565A2024 BNP PARIBAS ADR SPONSORED December 1, 2012 FR0000061129 BOIRON January 1, 2015 FR0000039299 BOLLORE August 1, 2012 FR0000120503 BOUYGUES August 1, 2012 US1021171087 -

Neia 20120906
Index Announcement Issue Date: Thursday 6 September 2012 Effective Date: Monday 24 September 2012 Announcement No: 2012-170 ______________________________________________________________________ Indices: Index ISIN Code CAC 40® FR0003500008 CAC Next 20® QS0010989109 CAC® Large 60 QS0011213657 SBF 120® FR0003999481 CAC® Mid 60 QS0010989117 CAC® Small QS0010989125 CAC® Mid & Small QS0010989133 CAC® All-Tradable FR0003999499 CAC® All-Share Index QS0010989141 Subject: Quarterly Review Index Adjustment: Changes in o compositions, (see appendix); o numbers of shares; o free float factors, (preliminary factors see appendix); o capping factors; o divisors. Conditions: The compiler retains the right to change the published selection in case of mergers, take-overs, suspension or resumption of trading during the period before the effective date of the review. Time table: After close of markets on Wednesday 19 September 2012 the following data will be published for each index and each constituent: o number of shares; o final free float percentage; o capping factor. After close of business on Friday 21 September 2012, the new divisor of the CAC 40 index will be published. Page 1 of 13 Avis Indice Date de publication : Jeudi 6 Septembre 2012 Date d’effet : Lundi 24 Septembre 2012 Numéro d’avis : 2012-170 ______________________________________________________________________ Indices: Indice Code ISIN CAC 40® FR0003500008 CAC Next 20® QS0010989109 CAC® Large 60 QS0011213657 SBF 120® FR0003999481 CAC® Mid 60 QS0010989117 CAC® Small QS0010989125 CAC® Mid & Small QS0010989133 CAC® All-Tradable FR0003999499 CAC® All-Share Index QS0010989141 Sujet: Révision trimestrielle Détails : Modifications o de la composition des indices, (voir l’annexe); o du nombre d’actions; o des flottants, (flottants préliminaires voir l’annexe); o des facteurs de plafonnement; o des diviseurs. -

Downloading the Video to Their Device (See Figure 3-63)
NORTHWESTERN UNIVERSITY Compositional Possibilities of New Interactive and Immersive Digital Formats A DISSERTATION SUBMITTED TO THE BIENEN SCHOOL OF MUSIC IN PARTIAL FULFILLMENT OF THE REQUIREMENTS for the degree DOCTOR OF MUSICAL ARTS Program of Composition By Daniel R. Dehaan EVANSTON, IL (June 2019) 2 Abstract From 2008 to 2019, a range of new interactive and immersive digital formats that present new possibilities for musical and artistic expression have become available. In order to begin the work of uncovering what new compositional and experiential possibilities are now possible, this document will examine each format’s core concepts and tools, cataloging the current state of related technologies. It also provides a survey of each format’s representative works, including a discussion of my original and evolving work for virtual reality, Infinite Void. The ultimate goal of this dissertation is to serve as a point of departure for composers interested in working with and influencing the direction that musical and creative expression will take in these immersive and interactive digital environments. 3 Acknowledgments This document would not have been possible without countless individuals to whom I owe more than just the acknowledgements of this page. To my committee members, Chris Mercer, Hans Thomalla, and Stephan Moore, who made themselves available from all corners of the globe and encouraged me to keep going even when it seemed like no end was in sight. To Donna Su, who kept me on track and moving forward throughout my entire time at Northwestern. To my readers, Nick Heinzmann and Caleb Cuzner, without whom I don’t think I would have ever been able to finish. -

Sony Interactive Entertainment Unveils Limited Edition Playstation®4 and Huge Discounts During the Days of Play Sale 2018
SONY INTERACTIVE ENTERTAINMENT UNVEILS LIMITED EDITION PLAYSTATION®4 AND HUGE DISCOUNTS DURING THE DAYS OF PLAY SALE 2018 Eleven days of incredible deals on hardware, games, services, peripherals and more kicks off on 8th June. May 29, 2018 – Sony Interactive Entertainment (SIE) today announced that Days of Play will be returning in 2018 offering gamers great savings across PlayStation® software, hardware and services. The Days of Play sale will take place from the 8th to the 18th June globally and this year includes incredible deals on a new PlayStation®4 (PS4™) Days of Play Limited Edition. Featuring the iconic PlayStation shapes in a dazzling gold design, this 500GB HDD PS4 comes with an additional DUALSHOCK®4 wireless controller. Within the Days of Play sale, PlayStation™Store (PS Store) are offering huge savings on AAA PS4 games from Sony Interactive Entertainment Worldwide Studios (SIE WWS) titles such as Horizon Zero Dawn™, Shadow of the Colossus and fastest-selling first party PS4 title God of War as well as third party titles such as Call of Duty®: WWII (Activision), Far Cry 5 (Ubisoft), Assassin's Creed® Origins (Ubisoft) and Grand Theft Auto V (Rockstar Games). In addition, the PlayStation®VR (PS VR) starter pack – which includes what gamers need for fully immersive gaming – is offered alongside fantastic deals on a range of PS VR software, including SIE WWS titles Farpoint and Starblood Arena. Other offers include PS4 software bundles, hardware peripherals, including a range of DUALSHOCK®4s and the Platinum Wireless Headset. Discounts will also apply to PlayStation services, including PlayStation®Plus (PS Plus) as well as on PlayStation®Gear, which offers a wide range of popular gaming merchandise, with new savings added throughout Days of Play.