Assessing the Prevalence of Urogenital
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Some Haematological Parameters Among Urinary Schistosomiasis-Malaria Coinfected Children in Suburb of Malentouen Health District, West Region Cameroon
International Journal of TROPICAL DISEASE & Health 41(7): 34-44, 2020; Article no.IJTDH.58309 ISSN: 2278–1005, NLM ID: 101632866 Some Haematological Parameters among Urinary Schistosomiasis-Malaria Coinfected Children in Suburb of Malentouen Health District, West Region Cameroon Keptcheu T. D. Leonard1*, Payne V. Khan1, Lehman Léopold Gustave2 and Gangue Tiburce3 1Department of Animal Biology, Faculty of Science, The University of Dschang, P.O. Box: 67, Dschang, Cameroon. 2Department of Animal Organisms, Faculty of Science, The University of Douala, P.O. Box: 24 157, Douala, Cameroon. 3Department of Biological Sciences, Faculty of Science, The University of Bamenda, P.O. Box: 39, Bambili, Bamenda, Cameroon. Authors’ contributions This work was carried out in collaboration among all authors. As part of PhD thesis, Author KTDL wrote the protocol, wrote the first draft of the manuscript, managed the literature searches and collected the data. Authors PVK and LLG designed the study, participated in the writing of the protocol and wrote the first draft of the manuscript. Author GT help in collecting data and performed the statistical analysis. All authors read and approved the final manuscript. Article Information DOI: 10.9734/IJTDH/2020/v41i730295 Editor(s): (1) Dr. Arthur V. M. Kwena, Moi University, Kenya. Reviewers: (1) Soheil Ebrahimpour, Babol University of Medical Sciences, Iran. (2) Jesús G. Rodríguez Diego, Universidad Autonoma Metropolitana (UAM), Mexico. Complete Peer review History: http://www.sdiarticle4.com/review-history/58309 Received 14 April 2020 Accepted 21 June 2020 Original Research Article Published 01 July 2020 ABSTRACT Background: Schistosomiasis and Malaria are among the most prevalent afflictions of humans who live in areas of poverty in the developing world. -
NW SW Presence Map Complete Copy
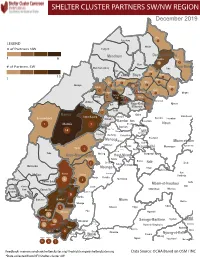
SHELTER CLUSTER PARTNERS SW/NWMap creation da tREGIONe: 06/12/2018 December 2019 Ako Furu-Awa 1 LEGEND Misaje # of Partners NW Fungom Menchum Donga-Mantung 1 6 Nkambe Nwa 3 1 Bum # of Partners SW Menchum-Valley Ndu Mayo-Banyo Wum Noni 1 Fundong Nkum 15 Boyo 1 1 Njinikom Kumbo Oku 1 Bafut 1 Belo Akwaya 1 3 1 Njikwa Bui Mbven 1 2 Mezam 2 Jakiri Mbengwi Babessi 1 Magba Bamenda Tubah 2 2 Bamenda Ndop Momo 6b 3 4 2 3 Bangourain Widikum Ngie Bamenda Bali 1 Ngo-Ketunjia Njimom Balikumbat Batibo Santa 2 Manyu Galim Upper Bayang Babadjou Malentouen Eyumodjock Wabane Koutaba Foumban Bambo7 tos Kouoptamo 1 Mamfe 7 Lebialem M ouda Noun Batcham Bafoussam Alou Fongo-Tongo 2e 14 Nkong-Ni BafouMssamif 1eir Fontem Dschang Penka-Michel Bamendjou Poumougne Foumbot MenouaFokoué Mbam-et-Kim Baham Djebem Santchou Bandja Batié Massangam Ngambé-Tikar Nguti Koung-Khi 1 Banka Bangou Kekem Toko Kupe-Manenguba Melong Haut-Nkam Bangangté Bafang Bana Bangem Banwa Bazou Baré-Bakem Ndé 1 Bakou Deuk Mundemba Nord-Makombé Moungo Tonga Makénéné Konye Nkongsamba 1er Kon Ndian Tombel Yambetta Manjo Nlonako Isangele 5 1 Nkondjock Dikome Balue Bafia Kumba Mbam-et-Inoubou Kombo Loum Kiiki Kombo Itindi Ekondo Titi Ndikiniméki Nitoukou Abedimo Meme Njombé-Penja 9 Mombo Idabato Bamusso Kumba 1 Nkam Bokito Kumba Mbanga 1 Yabassi Yingui Ndom Mbonge Muyuka Fiko Ngambé 6 Nyanon Lekié West-Coast Sanaga-Maritime Monatélé 5 Fako Dibombari Douala 55 Buea 5e Massock-Songloulou Evodoula Tiko Nguibassal Limbe1 Douala 4e Edéa 2e Okola Limbe 2 6 Douala Dibamba Limbe 3 Douala 6e Wou3rei Pouma Nyong-et-Kellé Douala 6e Dibang Limbe 1 Limbe 2 Limbe 3 Dizangué Ngwei Ngog-Mapubi Matomb Lobo 13 54 1 Feedback: [email protected]/ [email protected] Data Source: OCHA Based on OSM / INC *Data collected from NFI/Shelter cluster 4W. -

Predicted Distribution and Burden of Podoconiosis in Cameroon
Predicted distribution and burden of podoconiosis in Cameroon. Supplementary file Text 1S. Formulation and validation of geostatistical model of podoconiosis prevalence Let Yi denote the number of positively tested podoconiosis cases at location xi out of ni sample individuals. We then assume that, conditionally on a zero-mean spatial Gaussian process S(x), the Yi are mutually independent Binomial variables with probability of testing positive p(xi) such that ( ) = + ( ) + ( ) + ( ) + ( ) + ( ) 1 ( ) � � 0 1 2 3 4 5 − + ( ) + ( ) 6 where the explanatory in the above equation are, in order, fraction of clay, distance (in meters) to stable light (DSTL), distance to water bodies (DSTW), elevation (E), precipitation(Prec) (in mm) and fraction of silt at location xi. We model the Gaussian process S(x) using an isotropic and stationary exponential covariance function given by { ( ), ( )} = { || ||/ } 2 ′ − − ′ Where || ||is the Euclidean distance between x and x’, is the variance of S(x) and 2 is a scale − pa′rameter that regulates how fast the spatial correlation decays to zero for increasing distance. To check the validity of the adopted exponential correlation function for the spatial random effects S(x), we carry out the following Monte Carlo algorithm. 1. Simulate a binomial geostatistical data-set at observed locations xi by plugging-in the maximum likelihood estimates from the fitted model. 2. Estimate the unstructured random effects Zi from a non-spatial binomial mixed model obtained by setting S(x) =0 for all locations x. 3. Use the estimates for Zi from the previous step to compute the empirical variogram. 4. Repeat steps 1 to 3 for 10,000 times. -

REPUBLIQUE DU CAMEROUN Paix
REPUBLIQUE DU CAMEROUN REPUBLIC OF CAMEROON Paix - Travail - Patrie Peace - Work - Fatherland MINISTRY OF MINES, INDUSTRY AND TECHNOLOGICAL DEVELOPMENT STATISTICAL YEARBOOK 2017 OF THE MINES, INDUSTRY AND TECHNOLOGICAL DEVELOPMENT SUBSECTOR FOREWORD The Ministry of Mines, Industry and Technological Development is pleased to present the statistical yearbook of the Mines, Industry and Technological Development sub-sector, MIDTSTAT, 2017edition. Its aim is to put at the disposal of actors of the sub-sector on the one hand, and of the general public, on the other hand, statistical data which illustrate the actions and development policies implemented in this ministerial department. The document presents a series of statistical information relating, on the one hand, to contextual data on Cameroon and, on the other hand, to the mines, industry and technological development components. I, therefore, invite all potential users of the information contained in this valuable document to make good use of it, and to generate actions likely to further improve the performance of our sub-sector. I would like to seize this opportunity to express my appreciation to all those who have, in one way or another, contributed towards elaborating this document, in particular the National Institute of Statistics for its support, the central and devolved services of MINMIDT, structures under its supervisory authority, as well as the administrations called upon to provide statistical information. We welcome suggestions and contributions likely to enrich and improve -

Case Studies from Adamawa (Cameroon-Nigeria)
Open Linguistics 2021; 7: 244–300 Research Article Bruce Connell*, David Zeitlyn, Sascha Griffiths, Laura Hayward, and Marieke Martin Language ecology, language endangerment, and relict languages: Case studies from Adamawa (Cameroon-Nigeria) https://doi.org/10.1515/opli-2021-0011 received May 18, 2020; accepted April 09, 2021 Abstract: As a contribution to the more general discussion on causes of language endangerment and death, we describe the language ecologies of four related languages (Bà Mambila [mzk]/[mcu], Sombә (Somyev or Kila)[kgt], Oumyari Wawa [www], Njanga (Kwanja)[knp]) of the Cameroon-Nigeria borderland to reach an understanding of the factors and circumstances that have brought two of these languages, Sombә and Njanga, to the brink of extinction; a third, Oumyari, is unstable/eroded, while Bà Mambila is stable. Other related languages of the area, also endangered and in one case extinct, fit into our discussion, though with less focus. We argue that an understanding of the language ecology of a region (or of a given language) leads to an understanding of the vitality of a language. Language ecology seen as a multilayered phenom- enon can help explain why the four languages of our case studies have different degrees of vitality. This has implications for how language change is conceptualised: we see multilingualism and change (sometimes including extinction) as normative. Keywords: Mambiloid languages, linguistic evolution, language shift 1 Introduction A commonly cited cause of language endangerment across the globe is the dominance of a colonial language. The situation in Africa is often claimed to be different, with the threat being more from national or regional languages that are themselves African languages, rather than from colonial languages (Batibo 2001: 311–2, 2005; Brenzinger et al. -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -

Le Journal Des
République Du Cameroun Republic Of Cameroon Paix - Travail - Patrie Peace - Work - Fatherland Le journal des Bulletin d’Annonces des Marchés Publics / Public Contracts Bulletin - Directeur de Publication Joseph NGO SANTE PUBLIQUE 05 INFRASTRUCTURE 02 BATIMENTS ET TRAVAUX PUBLICS 06 EDUCATION 03 SOCIAL 03 TRANSPORT 03 ADMINISTRATION 07 ECONOMIE 02 RURAL 25 N° 2320 4 Juin 2021 4 June 2021 B.P: 6604 Yaoundé - Cameroun E-mail : [email protected] Fax : 222 206 043 / 222 203 326 Internet : www.armp.cm 5:50 PM/17H:50 Tél : 222 201 803 / 222 200 008 / 222 200 009 JOURNAL DES MARCHES PUBLICS SOMMAIRE # RESUME DES CONSULTATIONS Reference 017/AONO/C-BGOU/ST/CIPM-AI/2021 Lire POUR LES TRAVAUX D'ELECTRIFICATION DU VILLAGE DEMLOUP DANS LA COMMUNE DE BANGOU EN PROCÉDURE Titre/objet D'URGENCE.FINANCEMENT : BIP-RT-EXERCICE 2021 1 Nature de prestation Autres Infrastructures Date de cloture 18-06-2021 Reference N°007/C/C-NTUI/SG/CIPM/2021 Lire COMMUNIQUE N°007/C/C-NTUI/SG/CIPM/2021 DU 25/05/2021 PORTANT PUBLICATION DU RESULTAT DE L’APPEL D’OFFRES NATIONAL OUVERT N°004/AONO/ C-NTUI/SG/CIPM/2021 DU 23 MARS 2021 EN PROCEDURE D’URGENCE Titre/objet POUR LES TRAVAUX DE CONSTRUCTION D’UNE TRIBUNE AU STADE MUNICIPAL DE NTUI, DANS LA COMMUNE DE 2 NTUI, DEPARTEMENT DU MBAM ET KIM, REGION DU CENTRE Nature de prestation Bâtiments et Equipements Collectifs Date de cloture N/A Reference 008/D/C-DDG/SG/SIGAMP/CIPMP-DDG/2021 Lire PORTANT ATTRIBUTION DU MARCHE OBJET DE L'APPEL D'OFFRES NATIONAL OUVERT N° 04 /AONO/CDDG/ SG/SIGAIVLP/CIPMP-DDG/2021 DU 28/04/2021 POUR LA CREATION ET EXPLOITATION Titre/objet D'UNE CARRIERE SEMI MECANISEE A MVUH DANS LA COMMUNE DE DEMDENG, DEPARTEMENT DU KOUNG-KHI, 3 REGION DE L'OUEST {EN PROCEDURE D'UEGENCE) : PHASE 1. -

Republique Du Cameroun Ministere De L'energie Et De L'eau Contrat D'affermage Du Service Public De L'alimentation En Eau
REPUBLIQUE DU CAMEROUN MINISTERE DE L'ENERGIE ET DE L’EAU CONTRAT D'AFFERMAGE DU SERVICE PUBLIC DE L’ALIMENTATION EN EAU POTABLE DES CENTRES URBAINS ET PERIURBAINS DU CAMEROUN SOMMAIRE TITRE I – RÉGIME DE L'AFFERMAGE............................................................................... 6 Article 1 - Définitions ..............................................................................................................6 Article 2 - Valeur de l’exposé et des annexes..........................................................................9 Article 3 - Objet du Contrat d'Affermage..................................................................................9 Article 4 - Objet de l’Affermage...............................................................................................9 Article 5 - Définition du Périmètre d'Affermage......................................................................10 Article 6 - Révision du Périmètre d'Affermage.......................................................................10 Article 7 - Biens de retour .....................................................................................................10 Article 8 - Régime de Biens de Retour..................................................................................12 Article 9 - Renouvellement des Biens de Retour ...................................................................13 Article 10 - Inventaire des Biens de Retour.............................................................................13 Article 11 - Biens -

Hydropower in Cameroon
Hydropower in Cameroon DEPARTMENT OF BUILDING, ENERGY AND ENVIRONMENTAL ENGINEERING Hydropower in Cameroon Sebastien Tchuidjang Tchouaha Bachelors Thesis in Energy System Engineering Examiner: Ulf Larsson Supervisor: Ulf Larsson Page | 1 Hydropower in Cameroon Page | 2 Hydropower in Cameroon Acknowledgment Firstly, this thesis topic Hydropower in Cameroon has finally come to an end after some months of hard work, studies and research from different articles and books. Secondly, am thankful for having the opportunity to write on this topic with the help and approval of my head of department Ulf Larsson who has being of grate help and support throughout these past months. Furthermore, I will like to thank Belinda Fonyam my Fiancé, Yvonne a friend and some of my family members back home (Cameroon) for their assistance and support during this past months. Finally, I admit with gratitude the contribution of the scholars, journals, presses, the publishers of the article Global Village Cameroon (GVC), Mr. Kum Julius, Mr. Jie Cai, Mr. Egbe Tah Franklin, the ministry of Water and Energy (MINEE) and my brother Mr. Ndontsi Marcel a teacher in Cameroon whom has been instrumental during my research. Page | 3 Hydropower in Cameroon Page | 4 Hydropower in Cameroon Abstract Now our days with the great development of science and technology, it‟s of more importance to develop renewable energy sources used for electricity generation. I wish to introduce something about the Cameroonian water resources. Hydropower being one of the most used source of energy production in the world it has also developed rapidly in Cameroon whereby about 90% of the electricity generated is from hydropower and it also help in bursting the country‟s economy by exportation to neighbouring countries. -

The State of Cameroon's Biodiversity for Food and Agriculture
COUNTRY REPORTS THE STATE OF CAMEROON’S BIODIVERSITY FOR FOOD AND AGRICULTURE This country report has been prepared by the national authorities as a contribution to the FAO publication, The State of the World’s Biodiversity for Food and Agriculture. The report is being made available by the Food and Agriculture Organization of the United Nations (FAO) as requested by the Commission on Genetic Resources for Food and Agriculture. The information in this report has not been verified by FAO, and the content of this document is entirely the responsibility of the authors, and does not necessarily represent the views of FAO, or its Members. The designations employed and the presentation of material do not imply the expression of any opinion whatsoever on the part of FAO concerning legal or development status of any country, territory, city or area or of its authorities or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed by FAO in preference to others of a similar nature that are not mentioned. MINISTRY OFAGRICULTURE AND RURAL DEVELOPMENT (MINADER) THE STATE OF BIODIVERSITY FOR FOOD AND AGRICULTURE IN CAMEROON November 2015 MINISTRY OFAGRICULTURE AND RURAL DEVELOPMENT (MINADER) THE STATE OF BIODIVERSITY FOR FOOD AND AGRICULTURE IN CAMEROON November 2015 i CITATION This document will be cited as: MINADER (2015). The State of Biodiversity for Food and Agriculture in the Republic of Cameroon. -

The Infl Constituent in the Mundani Language
SCHOOL OF ORIENTAL AND AFRICAN STUDIES UNIVERSITY OF LONDON THE INFL CONSTITUENT IN THE MUNDANI LANGUAGE by Elizabeth Ann Magba A Thesis Submitted in Partial Fulfilment of the Requirements of the Degree of Doctor of Philosophy May, 1995 1 ProQuest Number: 10731691 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10731691 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ABSTRACT The thesis is a syntactic study of the Inflectional constituent (INFL) in Mundani, a Grassfields Bantu language of the Mbam-Nkam subgroup, spoken in the S.W. Province of the Republic of Cameroon. Chapter 1 b r ie f ly introduces the Mundani language and people; chapter 2 summarizes relevant aspects of the modular system of Universal Grammar known as Government and Binding (GB) theory that forms the basic theoretical framework of the study. In order to place the INFL constituent within its wider s y n ta c tic context, chapters 3 and 4 o u tlin e Mundani clause structure, including interrogatives, negation, and interesting deviations from basic SVO word order in Topic and Focus construction s. -

The Last Great Ape Organization, Cameroon Laga
THE LAST GREAT APE ORGANIZATION, CAMEROON LAGA ANNUAL REPORT JANUARY – DECEMBER 2014 Executive Summary Despite many obstacles, tangible achievements were made over this period in LAGA’s collaboration with MINFOF in the fields of investigation, arrest, prosecution, media exposure, government relations and international activities. Focus was on the fight against corruption, the illegal wildlife trade especially the specialized illicit trade in ape parts including fresh chimpanzee and gorilla heads, limbs and skulls; ivory and elephant products trafficking and the illicit trade in giant pangolin scales and leopards skins . The wildlife law enforcement operations carried out this year also busted international connections in ivory trafficking, ape parts and giant pangolin scales. This year, LAGA focused on the illegal trade in ape skulls uncovering an unprecedented magnitude of this specialized trade with 77 ape skulls seized and 25 traffickers arrested. 86% of those arrested stayed permanently behind bars from the moment of arrest and during the trial process while 10% were granted bail along the line and 4% were released and never prosecuted. Corruption was observed and fought in more than 80% of the cases. Operations focused on the illicit trade in ape parts and the trafficking in elephant bones and ivory while demonstrating the link between the two. 50 new cases were brought to court, 18 traffickers convicted and more than $326,000 to be paid as damages. Media exposure was at a rate of more than one media piece per day. Replication was concretized in Senegal - EAGLE replication, with SALF starting operations and AALF-B was launched in Benin with good operations.