Evaluation of the Relative Available Energy of Several Dietary Fiber Preparations Using Breath Hydrogen Evolution in Healthy Humans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
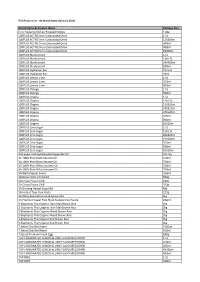
HCS Website List As of 31 Dec 2020.Xlsx
HCS Product List - By Brand Name (January 2021) Brand Name & Product Name Package Size (Lim Traders) Chicken Breaded Patties 1.8kg 100PLUS ACTIVE Non-Carbonated Drink 1.5L 100PLUS ACTIVE Non-Carbonated Drink 12X300ml 100PLUS ACTIVE Non-Carbonated Drink 300ml 100PLUS ACTIVE Non-Carbonated Drink 500ml 100PLUS ACTIVE Non-Carbonated Drink 6X300ml 100PLUS Blackcurrant 1.5L 100PLUS Blackcurrant 12X1.5L 100PLUS Blackcurrant 24X500ml 100PLUS Blackcurrant 500ml 100PLUS Hydration Bar 4X75ml 100PLUS Hydration Bar 75ml 100PLUS Lemon Lime 1.5L 100PLUS Lemon Lime 325ml 100PLUS Lemon Lime 500ml 100PLUS Orange 1.5L 100PLUS Orange 500ml 100PLUS Original 1.5L 100PLUS Original 12X1.5L 100PLUS Original 12X325ml 100PLUS Original 24X325ml 100PLUS Original 24X500ml 100PLUS Original 325ml 100PLUS Original 500ml 100PLUS Original 6X325ml 100PLUS Zero Sugar 1.5L 100PLUS Zero Sugar 12X1.5L 100PLUS Zero Sugar 24X325ml 100PLUS Zero Sugar 24X500ml 100PLUS Zero Sugar 325ml 100PLUS Zero Sugar 500ml 100PLUS Zero Sugar 6X325ml 333 Super Refined Blended Vegetable Oil 1X17kg 3A 100% Pure Black Sesame Oil 320ml 3A 100% Pure Black Sesame Oil 750ml 3A 100% Pure White Sesame Oil 320ml 3A 100% Pure White Sesame Oil 750ml 3A Black Pepper Sauce 250ml 3A Brown Rice Vermicelli 500g 3A Crispy Prawn Chilli 180g 3A Crispy Prawn Chilli 320g 3A Ginseng Herbal Soup Mix 40g 3A Instant Tom Yum Paste 227g 3A Klang Bakuteh Herbs & Spices Mix 35g 3A Premium Sugar Free Black Soybean Soy Sauce 400ml 3-Elephants Thai Organic Hom Mali Brown Rice 1kg 3-Elephants Thai Organic Hom Mali Brown Rice 2kg -

Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles
Foreign Agricultural Service GAIN Report Global Agriculture Information Network Approved by: Date: 07/23/99 Sarah D. Hanson GAIN Report #JA9087 U.S. Embassy Market Brief - Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles This report was prepared by the USDA’s Foreign Agricultural Service for U.S. exporters of food and agricultural products. This information is in the public domain and may be reprinted without permission. Use of commercial or trade names does not imply approval nor constitute endorsement by USDA/FAS. Tokyo[JA1], JA GAIN Report #JA9087 Page 1 of 24 Company Name Amway Japan Product Sector(s) Health and Functional Food Address 1-8-1, Shimo-Meguro Number Of Employees 728 Meguro-ku, Tokyo 153-8686 Number of Factories Overseas Contact Phone Number 03-5434-8484 Fax Number 03-5434-4923 Email Web Page Address www.amway.co.jp/amway_japan/ Contact Person Masura Iwata Executive Driector, External Affairs and Public Relations Sales and Net Profits Main Suppliers Year Sales (Mil. \) Net Profits 1995 177,991 22,424 1996 212,195 25,130 1997 203,361 26,638 Key Products % of Total Company Profile and Strategies Home Care Products 9 Japanese corporation of nonstore sales operator Amway (US). Housewares 30 Registered sales personnel involved in direct sales of detergents, Personal Care 34 cosmetics, kitchenware and nutritional supplements. Nutritional Supplements 23 Others 4 Main Brands Triple X (vitamin and mineral supplement), Nutri Protein, Acerola C (vitamin supplement), Salmon-Omega 3, Hon-E-Cece, Ironics, Beta Carotene A, Wheat Germ E. Main Ingredients Vitamins, protein concentrates, iron concentrates, calcium concentrates, beta caroten, wheat germ. -

ANNUAL REPORT 2016 Corporate Philosophy
ANNUAL Otsuka Holdings Co., Ltd. ANNUAL REPORT 2016 REPORT 2016 For the year ended December 31, 2016 Corporate Philosophy Creating innovative products These words embody our commitment to: Contributing Improving to the human health lives of people worldwide In keeping with this corporate philosophy and the Otsuka mottos of Jissho (Actualization) and Sozosei (Creativity), the Otsuka Group strives to utilize its unique resources and skills to develop differentiated scientific solutions which contribute to the lives of people worldwide in the form of innovative and creative products ranging from pharmaceuticals to consumer products. We are striving to cultivate a culture and a dynamic corporate climate reflecting our vision as a healthcare company. Consistent with this approach, we are dedicated to achieving global sustainability, to our relationships with local communities, and to the protection of the natural environment. Contents About Otsuka Creating Corporate Value Corporate Philosophy Corporate Governance ............. 42 Directors, Audit & Supervisory Business Model ......................... 02 Board Members ........................ 48 Corporate Social Business Segments ................... 04 Responsibility ........................... 50 Financial and Non-Financial Finance & General Information Highlights ................................. 06 Financial Summary ................... 54 Consolidated Financial Statements ................ 56 Message from the President ..... 08 Group Structure & Overview of Main Operating Companies ..... -

Weekend Special
THE NEST WEEKEND SPECIAL JUNE 6 & 7, 2020 Member Standard JUNE 13 & 14, 2020 Member Standard BRAISED KAMPUNG 22.5 24.5 ‘KUNG PAO’ PRAWN 22.5 24.5 SPRING CHICKEN (HALF) Sautéed Prawn with Dried Chilli, Onion, Ginger, Spring Marinated with Dark Soy Sauce, Japanese Shoyu and Onion, Tri-Colour Capsicum Garnished with Cashew Nuts Ginger, Slight Deep-Fried then Braised with Garlic, Shallot, Ginger, Leek, Superior Oyster Sauce and Ginseng Roots LEMON CHICKEN CUTLET 15.5 16.5 Deep-Fried Boneless Chicken Thigh Coated with OATMEAL SEABASS 22.5 24.5 Breadcrumbs Accompanied with Homemade Lemon Sauce Crispy Seabass Fillet Stir-Fried with Oatmeal, Curry Leaves, Chilli Padi and Margarine BLACK GAROUPA (WHOLE) 32.5 34.5 Deep-Fried Black Garoupa Served with Caramelized Onion SALTED EGG YOLK BITTER GOURD 12.5 13.5 and Dark Sauce Sautéed Bitter Gourd with Salted Egg Yolk All prices are in Singapore dollars and subject to prevailing government tax PLEASE CALL +65 6248 1711 FOR ORDERS SELF-COLLECT AND DELIVERIES AVAILABLE 10% OFF SELF-COLLECT ORDERS OPERATING HOURS: 11AM TO 7PM 11 LAGUNA GOLF GREEN SINGAPORE 488047 THE NEST WEEKEND SPECIAL FATHER’S DAY LUCKY DRAW PROMOTION Every $58 spent (single receipt, before GST) in June entitled one entry for our lucky draw! Prize worth more than $1,000 includes: One round of Weekday Golf at Masters Course for two persons Two $58 F&B vouchers One bottle of Lagavulin 16 Years Single Malt Scotch Whisky *Preorder required. Last order on June 18, 2020 before 3.00pm JUNE 20 & 21, 2020 FATHER’S DAY SPECIALS ADD-ONS (SERVING FOR 4 – 6 PERSONS) Member Standard (SERVING FOR 4 – 6 PERSONS) Member Standard * ROASTED DUCK – CHINESE STYLE 58 68 * ASIAN SIDE DISHES 38 48 (WHOLE) Mixed Salad Greens with Kombu Dressing, Stir-Fried Roasted Duck Served with Plum Sauce Kai Lan with Mushrooms and Chef’s Special Fried Rice * ROASTED BEEF BRISKET (APPROX. -

Japan Energy and Sports Drink Market: Sample Report
1 © This is a licensed product of Ken Research and should not be copied TABLE OF CONTENTS 1. Asia Energy and Sports Drinks Market Introduction 2. Asia Energy and Sports Drinks Market Size, 2007-2012 3. Japan Energy and Sports Drinks Market Introduction 4. Japan Energy and Sports Drinks Market Size, 2007-2012 5. Japan Energy and Sports Drinks Market Segmentation by Functionality, 2007-2012 5.1. For Consumers at Work, 2007-2017 5.2. For Consumers at Play, 2007-2017 5.3. For Consumers at Leisure, 2007-2017 6. Japan Quasi Drug Energy Drink Market Segmentation by Distribution Channel, 2007-2012 6.1. For Drug Stores, 2007-2017 6.2. For Convenience Stores, 2007-2017 6.3. For Supermarkets and Hypermarkets, 2007-2017 6.4. For Others (Small Independent Retailers and Conventional Grocery Stores), 2007-2017 7. Japan Energy Drink Market Segmentation by Distribution Channel, 2007-2012 7.1. For Vending Machines, 2007-2017 7.2. For Convenience Stores, 2007-2017 7.3. For Supermarkets and Hypermarkets, 2007-2017 7.4. For Others (Small Independent Shops and Retailers), 2007-2017 8. Japan Sports Drink Market Segmentation by Distribution Channel, 2007-2012 8.1. For Hypermarkets and Supermarkets, 2007-2017 8.2. For Convenience Stores, 2007-2017 8.3. For Vending Machines, 2007-2017 8.4. For Others (Small Independent Retailers and Grocery Stores), 2007-2017 9. Japan Energy and Sports Drinks Market Trends and Developments Surge in the Number of Vending Machines in Japan Increasing Number of Fitness Clubs and Programs 2 © This is a licensed product of Ken Research and should not be copied Increase in the Expenditure on Food and Beverage Products 10. -

Part 3 TRADITIONAL JAPANESE CUISINE
Part 3 TRADITIONAL JAPANESE CUISINE Chakaiseki ryori is one of the three basic styles of traditional Japanese cooking. Chakaiseki ryori (the name derives from that of a warmed stone that Buddhist monks placed in the front fold of their garments to ward off hunger pangs) is a meal served during a tea ceremony. The foods are fresh, seasonal, and carefully prepared without decoration. This meal is then followed by the tea ceremony. (Japan, an Illustrated Encyclopedia , 1993, p. 1538) Honzen ryori is one of the three basic styles of traditional Japanese cooking. Honzen ryori is a highly ritualized form of serving food in which prescribed types of food are carefully arranged and served on legged trays (honzen). Honzen ryori has its main roots in the so- called gishiki ryori (ceremonial cooking) of the nobility during the Heian period (794 - 1185). Although today it is seen only occasionally, chiefly at wedding and funeral banquets, its influence on modern Japanese cooking has been considerable. The basic menu of honzen ryori consists of one soup and three types of side dishes - for example, sashimi (raw seafood), a broiled dish of fowl or fish (yakimono), and a simmered dish (nimono). This is the minimum fare. Other combinations are 2 soups and 5 or 7 side dishes, or 3 soups and 11 side dishes. The dishes are served simultaneously on a number of trays. The menu is designed carefully to ensure that foods of similar taste are not served. Strict rules of etiquette are followed concerning the eating of the food and drinking of the sake. -

SNACKS & RAW • スナックス・ロー Qty Pacific Oyster 4Ea Szechuan
SNACKS & RAW • スナックス・ロー Qty Pacific oyster 4ea Szechuan pickles 6 Typhoon shelter school prawns 10 Raw sea bream, fresh wasabi, ginger & nashi 15 Raw cobia, orange dashi & finger lime 18 SALAD & VEGETABLES • サラド・ベジタブルズ Butter lettuce, many herbs, shiso & kombu 12 Tare pumpkin, dashi cream, walnut & laver 15 Roasted mushrooms, cashews, artichoke & noodles 15 PLATES •プレーツ Sweet corn congee, smoked butter & nori 10 House cured Wagyu brisket, Szechuan pepper 15 New England lobster roll 17 Grilled calamari, XO & coastal herbs 17 FROM THE HIBACHI • フロム・ヒバーチー King brown mushroom, tare & sansho pepper 5ea Chicken liver yakitori 6ea Corn fed chicken yakitori — thigh / tsukune 7ea S and whiting, yuzu kosho 6ea Char siu pork neck, garlic chives 16 DUMPLING & BAO • ダンプリング・バウ Vegetable dumplings, shiso dressing (5 pce) 13 Prawn & chicken dumplings, chilli sauce (5 pce) 15 Northern style cumin lamb, pancakes & pickles 24 Twice-cooked duck leg, vinegar & plum sauce, bao 29 LARGER DISHES • ラージャー・ディシズ Whole pan roasted flounder, kombu & burnt butter 34 Half roast chicken, smoked eggplant & spring onion 36 WE ARE NOW OPEN FOR SUNDAY LUNCH BANQUET • バンケット(65PP) Szechuan pickles Raw sea bream, fresh wasabi, ginger & nashi Tare pumpkin, dashi cream, walnut & laver New England lobster roll Roasted mushrooms, cashews, artichoke & noodles Prawn & chicken dumplings, chilli sauce Northern style cumin lamb, pancakes & pickles Peanut butter parfait, salted caramel, chocolate DRINK WITH YAKITORI • カクテルやスピリッツ Asahi Super Dry 10 Asahi Dry Black 12 Four Pillars gin -

Beer House Sake Plum Wine Premium Cold Sake Mini Bottle Sake
premium cold sake (Masu Cap 85ml) Masu Cup Bottle Yaegaki Dry 15.0% £ 3.50 £ 24.00 A full bodied palate which has a slight acidity. (750ml) Dassai Junmai Dai-Ginjo 16% £ 5.60 £ 45.00 Amazing floral nose and a delicate balance of (720ml) sweetness with notes of sweet melon and drink apples. Dassai Nigori Junmai (unfiltered) 16.5% £ 5.00 An exhilarating nose of cream and spices and a textured palate with a slight sweetness. Suishin Junmai Ginjo 15.5% £ 5.40 £ 44.00 Medium dry sake with a mild fragrance and great (720ml) depth. Shirakabegura Muroka Genshu Dai-Ginjo 17% £ 6.80 £ 48.00 Premium unfiltered Daiginjo made from the high (640ml) quality rice. Fresh ginjo aroma that hints of apple, smooth and rich flavour. mini bottle sake beer (300ml) (Bottle 330ml) Gokai Nama Sake 13% - 14% £ 9.80 Nama is a draft-style sake that is not heat pasteurized but rather micro-filtered. Asahi Super Dry 5% £ 3.50 Characterized by its fresh, smooth and very dry. This sake perfect with sashimi Kirin Ichiban 5% £ 3.50 and teriyaki food. Sapporo Premium 4.7% £ 3.50 Kasumitsuru Kimoto 16% £ 18.00 Smoky, earthy flavours mix with sweeter, fruitier notes in this warm and plum wine layered sake. Enjoy chilled or gently warmed. Slightly sweet. Glass Bottle Dassai Nigori Junmai (unfiltered) 15% £ 16.00 Plum Wine ABV 10% £ 4.50 (75ml) £ 29.50 (750ml) An exhilarating nose of cream and spices and a textured palate with a slight (Plum wine is served with crushed ice) sweetness. house sake Mio Sparkling Sake 5% £ 14.00 House Hot Sake (Tokkuri, ABV 15.3%, 200ml) £ 3.80 This new style sparkling sake is perfectly balanced in taste and flavour with House Hot Sake (Tokkuri, ABV 15.3%, 300ml) £ 5.60 moderate acidity and pleasant sweetness by our unique maturation in cool temperature. -

Pacific Lounge
PACIFIC LOUNGE 1 NON ALCOHOLIC DRINKS JUICE AND SOFT DRINK soft drink – pepsi, coke zero, diet coke, 7up, ginger ale, tonic 60 pocari sweat 60 chilled juice – orange, pineapple, cranberry, guava or apple 60 fresh juice – orange 80 watermelon, honey dew, rock melon, carrot 70 celery or tomato kratingdaeng 65 MINERAL WATER Natural equil natural 380ml 68 evian natural 500ml 75 equil natural 760ml or acqua panna 500ml 85 acqua panna 750ml 98 Sparkling perrier 330ml 75 equil sparkling 380ml 68 equil sparkling 760ml 85 san pellegrino 500ml 85 san pellegrino 750ml 98 SIGNATURE MOCKTAIL jaipur – mint, guava, lime, lychee 75 NON-ALCOHOLIC MOCKTAIL magenta – blueberry, kaffir, cranberry juice, passion fruit, seven up 75 kiwi passionate – lime, kaffir lime, kiwi, orange juice, 75 passion syrup, seven up summer shower – green apple, lime, sugar, peppermint tea 75 HOME MADE LEMONADE lemonade – lemon juice, sugar, water 70 pink lemonade – lemon juice, sugar, cranberry juice, water 70 mint lemonade – lemon juice, sugar, mint leaf, water 70 ginger lemonade – lemon juice, sugar, ginger, water 70 apple lemonade – lemon juice, sugar, green apple, water 70 all prices in rupiah one thousand, subject to 21% service and government tax PACIFIC 2 LOUNGE COFFEE & TEA HOUSE BREWS coffee – regular or decaffeinated, espresso 65 cappuccino or café latte oversized cappuccino 75 french earl grey – black tea, grand classic – earl grey 70 english breakfast – black tea, classic tea for morning 70 1837 black tea - exclusive tea blends 70 pai mu tan - white tea, china , -

GAIN Report Global Agriculture Information Network
Foreign Agricultural Service GAIN Report Global Agriculture Information Network Approved by: Date: 07/28/99 Sarah D. Hanson GAIN Report #JA9089 U.S. Embassy Market Brief Japan : Food Processing Sector - Retort Pouch Food Company Pofiles This report was prepared by the USDA’s Foreign Agricultural Service for U.S. exporters of food and agricultural products. This information is in the public domain and may be reprinted without permission. Use of commercial or trade names does not imply approval nor constitute endorsement by USDA/FAS. Tokyo[JA1], JA GAIN Report #JA9089 Page 1 of 36 Company Name Ajinomoto Co., Inc. Product Sector(s) Soup, Frozen Food, Retort Pouch Address 1-15-1, Kyobashi, Chuo-ku Number Of Employees 5,319 Tokyo 104 Number of Factories 5 Overseas Contact Phone Number 03-5250-8111 Fax Number 03-5250-8378 American Head Office Email Glenpointe Cetre West Web Page Address http://www.ajinomoto.co.jp/ 500 Frank W. Burr Blvd. Contact Person Norio Yamaguchi, Managing Director, Processed Foods Teaneck, N.J. 07666-6994 Division Tel: 201-488-1212 Sales and Net Profits Main Suppliers Year Sales (Mil. \) Net Profits 1995 580,260 7,534 Itohchu Shoji, Mitsubishi Shoji, Marubeni, Knorr Shokuhin, 1996 597,069 10,118 Calpis Shokuhin Kogyo 1997 613,102 10,261 Key Products % of Total Company Profile and Strategies Seasonings 19 Largest seasoning maker in Japan and ranks among the Oils and Fats 12 world's leaders in advanced amino acid application technology. Processed Foods 26 Beverages and Dairy Products 28 Sales of frozen foods, soups and retort packaged foods Pharmaceuticals, Amino Acids, Chemicals 11 are growing. -

Explain About Enkai in General & Subsequent Parties
Explain about enkai in general & subsequent parties following. Itadakimasu and Gochisōsama "Itadakimasu" ("I gratefully receive") before starting to eat "Gochisōsama (deshita)" ("Thanks for the food") after finishing the meal Usually accompanied by putting your hands together -but no religious connotation General etiquette for eating ● After finishing eating, try to place all your dishes in the same way as they were at the start of the meal. This includes replacing the lid of dishes which came with a lid and replacing your chopsticks on the chopstick holder or into their paper cover, if applicable. Rice: The rice is brought to your mouth, rather than the bowl, but it’s OK to lift your bowl closer to you while eating your rice. Do not pour soy sauce over rice. It is usually considered polite to finish all but a few grains of rice in the bowl. Sushi : If you choose to get sushi without wasabi you can use the phrase “wasabi nuki de..” In general, you are supposed to eat a sushi piece in one bite. Hands or chopsticks can be used to eat sushi. Just be sure not to leave any grains of rice behind. In case of nigiri-zushi (the one with the fish on top), dip the fish part into the soy sauce. A few kinds of nigiri-zushi, for example, egg or eel, should not be dipped into soy sauce. In case of maki-zushi (sushi wrapped in seaweed), just dip the bottom bit if desired before eating. Miso Soup: Drink the soup directly out of the bowl bring the bowl by bringing it to your mouth. -

S Ta R T E R S O
S T A R T E R S O U P HANDARA PRAWN COCKTAIL............80 BEEF GOULASH.................................... 55 Boiled fresh prawn, Lettuce, Hungarian beef stew and vegetable soup tomato and egg with cocktail sauce with garlic bread FRESH TOMATO BRUCHETTA....................50 FRESH CORN SOUP..............................45 Dice of fresh tomato, onion, Pure of fresh corn with garlic bread red chili, coriander leave, parmesan, olive oil on bed of crispy baguette CLEAR CHICKEN AND VEGETABLES SOUP 45 Carrot, string bean, cabbage, cauliflower, tomato, VEGETABLES SPRING ROLLS.............55 leek and dice of chicken Deep fried Mix julienne vegetables with garlic bread wrap with sweet and sour sauce SOTO AYAM...................................................75 GRILLED CHICKEN CAESAR SALAD...70 Indonesian favorite chicken soup, bean Baby romaine, beef bacon, egg, crouton sprout, glass noodle, with anchovy Caesar dressing tomato, egg served with rice SIGNATURE DISHES TOM YUM GOONG ...............................90 Thai style prawn soup, straw mushroom, HANDARA GARDEN SALAD................45 tomato, chili, fresh coriander and lime, Mix salad, tomato, onion, capsicum with citrus spicy and sour taste dressing and sharp parmesan cheese SANDWICHES & PASTA CHEESESTEAK HOAGIE.............................95 Thin slice of beef, mushroom, capsicum, onion and cheddar cheese in hot dog bun with fries and salad HANDARA BEEF BURGER...........................95 150 gr beef patty, caramelized onion, GADO GADO......................55 lettuce, tomato, beef bacon