ANNUAL REPORT 2016 Corporate Philosophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -
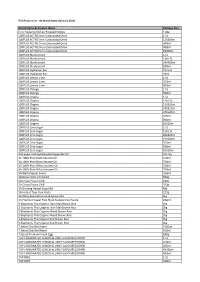
HCS Website List As of 31 Dec 2020.Xlsx
HCS Product List - By Brand Name (January 2021) Brand Name & Product Name Package Size (Lim Traders) Chicken Breaded Patties 1.8kg 100PLUS ACTIVE Non-Carbonated Drink 1.5L 100PLUS ACTIVE Non-Carbonated Drink 12X300ml 100PLUS ACTIVE Non-Carbonated Drink 300ml 100PLUS ACTIVE Non-Carbonated Drink 500ml 100PLUS ACTIVE Non-Carbonated Drink 6X300ml 100PLUS Blackcurrant 1.5L 100PLUS Blackcurrant 12X1.5L 100PLUS Blackcurrant 24X500ml 100PLUS Blackcurrant 500ml 100PLUS Hydration Bar 4X75ml 100PLUS Hydration Bar 75ml 100PLUS Lemon Lime 1.5L 100PLUS Lemon Lime 325ml 100PLUS Lemon Lime 500ml 100PLUS Orange 1.5L 100PLUS Orange 500ml 100PLUS Original 1.5L 100PLUS Original 12X1.5L 100PLUS Original 12X325ml 100PLUS Original 24X325ml 100PLUS Original 24X500ml 100PLUS Original 325ml 100PLUS Original 500ml 100PLUS Original 6X325ml 100PLUS Zero Sugar 1.5L 100PLUS Zero Sugar 12X1.5L 100PLUS Zero Sugar 24X325ml 100PLUS Zero Sugar 24X500ml 100PLUS Zero Sugar 325ml 100PLUS Zero Sugar 500ml 100PLUS Zero Sugar 6X325ml 333 Super Refined Blended Vegetable Oil 1X17kg 3A 100% Pure Black Sesame Oil 320ml 3A 100% Pure Black Sesame Oil 750ml 3A 100% Pure White Sesame Oil 320ml 3A 100% Pure White Sesame Oil 750ml 3A Black Pepper Sauce 250ml 3A Brown Rice Vermicelli 500g 3A Crispy Prawn Chilli 180g 3A Crispy Prawn Chilli 320g 3A Ginseng Herbal Soup Mix 40g 3A Instant Tom Yum Paste 227g 3A Klang Bakuteh Herbs & Spices Mix 35g 3A Premium Sugar Free Black Soybean Soy Sauce 400ml 3-Elephants Thai Organic Hom Mali Brown Rice 1kg 3-Elephants Thai Organic Hom Mali Brown Rice 2kg -

OCTOBER 2020 PTAB Public Hearing Schedule
OCTOBER 2020 PTAB Public Hearing Schedule Proceeding No. Serial No. PARTY Date Time 2020-004197 16043641 BIOGEN MA INC Thursday, October 1, 2020 9:00 AM (EDT) ALLERGAN PHARMACEUTICALS 2020-002635 13907447 Thursday, October 1, 2020 9:00 AM (EDT) INTERNATIONAL LIMITED 2020-002276 13612925 BAYER AG (MONSANTO) Thursday, October 1, 2020 9:00 AM (EDT) 2020-002277 13612929 OTSUKA PHARMACEUTICAL CO., 2020-002304 14341306 Thursday, October 1, 2020 9:00 AM (EDT) LTD. 2020-002327 15893354 CELLECTIS Thursday, October 1, 2020 9:00 AM (EDT) 2020-000258 15163285 POLY GROUP LLC Thursday, October 1, 2020 9:00 AM (PDT) 2020-000640 15414456 DISCERNDX INC. Thursday, October 1, 2020 9:00 AM (PDT) 2020-000934 13982470 DENOVO BIOMARKERS INC. Thursday, October 1, 2020 9:00 AM (PDT) THE BOARD OF TRUSTEES OF THE 2020-001247 15175848 LELAND STANFORD JUNIOR Thursday, October 1, 2020 9:00 AM (PDT) UNIVERSITY EDWARDS LIFESCIENCES 2020-001487 14563866 Thursday, October 1, 2020 9:00 AM (PDT) CORPORATION IKEDA FOOD RESEARCH COL LTD 2020-003630 16145178 Thursday, October 1, 2020 1:00 PM (EDT) and PHC CORPORATION 2020-002049 13310632 ADARE PHARMACEUTICALS INC. Thursday, October 1, 2020 1:00 PM (EDT) 2020-000053 13135898 OLEG ILLICH EPSHTEIN Thursday, October 1, 2020 1:00 PM (EDT) 2020-001829 15008646 MERZ PHARMA GMBH & CO. KGAA Thursday, October 1, 2020 1:00 PM (EDT) 2020-002360 15407037 IONIS PHARMACEUTICALS INC. Thursday, October 1, 2020 1:00 PM (EDT) 2020-002113 13729631 L'OREAL Thursday, October 1, 2020 1:00 PM (EDT) 2019-003658 13576565 QUNZHU LI and LI LI Thursday, October 1, 2020 1:00 PM (EDT) 2020-000322 14136660 CYTEC INDUSTRIES INC. -

Evaluation of the Relative Available Energy of Several Dietary Fiber Preparations Using Breath Hydrogen Evolution in Healthy Humans
J Nutr Sci Vitaminol, 60, 246–254, 2014 Evaluation of the Relative Available Energy of Several Dietary Fiber Preparations Using Breath Hydrogen Evolution in Healthy Humans Tsuneyuki OKU and Sadako NAKAMURA Graduate School of Human Health Science, University of Nagasaki, Siebold, Nagasaki 851–2195, Japan (Received December 9, 2013) Summary A standardized simple, indirect method for assessing the relative energy of dietary fiber carbohydrates is not yet established. There is a need for a standardized in vivo assay. The objective of the present study was to evaluate the relative available energy (RAE) for 9 major dietary fiber materials (DFMs) based on fermentability from breath hydrogen excretion (BHE) in subjects. Fructooligosaccharide (FOS) was used as a reference. The study was conducted using a within-subject, repeated measures design and approved by the Ethi- cal Committee of University of Nagasaki. After DFM ingestion, end-expiratory gas (750-mL) was collected at 1-h intervals for 8 h, as well as at 2-h intervals between 8 h and 14 h, and 30 min after waking up and 24 h after DFM ingestion. Breath hydrogen concentration was assessed with a gas chromatograph. The RAE of DFMs tested was evaluated based on the area under the curve (AUC) of BHE of FOS. Based on the ratio of AUC for 8 h, the RAE of polydextrose, partially hydrolysed guar gum, resistant maltodextrin and partially hydrolysed alginate was 1 kcal/g, and that of glucomannan, heat-moisture treatment and high-amy- lose cornstarch and cellulose was 0 kcal/g, while the RAE of all tested DEMs including cel- lulose and glucomannan was 1 kcal/g in the calculation based on AUCs for 14 h and 24 h in subjects. -

OSB Participant List by Research Area
OSB Participant List by Research Area Contact Centers (CC) • AARP • Air Products and • American Drug Stores Chemicals • AAA • ABB • American Electric Power • Airbus • Accor • Abbott • American Express • Alcatel Lucent • American Electric Power • Abengoa • American International • Alcoa Group • American International • Abu Dhabi National Group Energy Company • Alcon • American Stores Company • Austin Energy • ACC Limited • Alfa • American Water • Bank of America • Access Insurance Holdings • Algonquin Power & • Amgen Utilities • Blue Cross Blue Shield • Accord Holdings • AMIL • ALH Group • Charles Schwab & • ACE • AmInvestment Bank Company • Alitalia • Acea • AMR • Citigroup • ALK Abello • Acer • Amssi • Citizens Gas • Alkermes • Acxiom • Amtran Logistics • Clarke American • Allergan • Adelaide Clinic Holdings • Andrew Corporation • CPS Energy • Alliance & Leicester • Adidas • Anglian Water Services • Direct Energy • Alliance Boots • Advance Food Company • Anritsu • Federal Reserve Bank of • Alliant Techsystems Minneapolis • Advance Publications • Anschutz • Allianz • John Deere • Advanced Coating • Apache • Allied Irish Banks • Technologies Louisville Water Company • Apex Equity Holdings • Advanced Semiconductor • Allstate Insurance • Manila Electric Company Engineering Company • Apple • • • Mellon Financial Adventist Health System Ally Financial • Arcadia Housing • • • MetLife Aegon Alon USA Energy • Arcos Dorados Holdings • • • Morgan Stanley AEON AlpTransit Gotthard • Ardent Health Services • • • NetBank Aera Energy Alstom • Argos • -

Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles
Foreign Agricultural Service GAIN Report Global Agriculture Information Network Approved by: Date: 07/23/99 Sarah D. Hanson GAIN Report #JA9087 U.S. Embassy Market Brief - Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles This report was prepared by the USDA’s Foreign Agricultural Service for U.S. exporters of food and agricultural products. This information is in the public domain and may be reprinted without permission. Use of commercial or trade names does not imply approval nor constitute endorsement by USDA/FAS. Tokyo[JA1], JA GAIN Report #JA9087 Page 1 of 24 Company Name Amway Japan Product Sector(s) Health and Functional Food Address 1-8-1, Shimo-Meguro Number Of Employees 728 Meguro-ku, Tokyo 153-8686 Number of Factories Overseas Contact Phone Number 03-5434-8484 Fax Number 03-5434-4923 Email Web Page Address www.amway.co.jp/amway_japan/ Contact Person Masura Iwata Executive Driector, External Affairs and Public Relations Sales and Net Profits Main Suppliers Year Sales (Mil. \) Net Profits 1995 177,991 22,424 1996 212,195 25,130 1997 203,361 26,638 Key Products % of Total Company Profile and Strategies Home Care Products 9 Japanese corporation of nonstore sales operator Amway (US). Housewares 30 Registered sales personnel involved in direct sales of detergents, Personal Care 34 cosmetics, kitchenware and nutritional supplements. Nutritional Supplements 23 Others 4 Main Brands Triple X (vitamin and mineral supplement), Nutri Protein, Acerola C (vitamin supplement), Salmon-Omega 3, Hon-E-Cece, Ironics, Beta Carotene A, Wheat Germ E. Main Ingredients Vitamins, protein concentrates, iron concentrates, calcium concentrates, beta caroten, wheat germ. -

A-1256 Withdrawal of Thiopurines in Crohn's Disease Treated
Conflict of Interest T. HISAMATSU. Honoraria: EA pharma Co. Ltd., AbbVie GK, Celgene K.K., Janssen Pharmaceutical K.K., Pfizer Inc., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Mochida Pharmaceutical Co., Ltd., Nichi-lko Pharmaceutical Co., Ltd. Commercial research funding: EA pharma Co. Ltd., AbbVie GK, Daiichi-Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., Mochida Pharmaceutical Co., Ltd, Nippon Kayaku Co. Ltd., Kyorin Pharmaceutical Co. Ltd., JIMRO Co. Mochida Pharmaceutical Co., Ltd., Astellas Pharma Inc., Asahi Kasei Medical Co., Ltd., ZERIA Pharmaceutical Co. Ltd. S. KATO. Honoraria: Mistubishi Tanabe Pharma Corporation , Janssenn Pharma K,K. R. KUNISAKI. Honoraria: AbbVie GK, EA pharma Co. Ltd., Janssen Pharmaceutical K.K., JIMRO Co. Ltd., Kissei Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Nioppon Kayaku Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co. Ltd., ZERIA Pharmaceutical Co. Ltd. Commercial research funding: AbbVie GK, EA pharma Co. Ltd., Janssen Pharmaceutical K.K., JIMRO Co. Ltd., Kissei Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Corporation, RPM Co. Ltd, Takeda A-Pharmaceutical1256 Co. Ltd. M. MATSUURA. Honoraria: AbbVie GK, Mitsubishi Tanabe Pharma Corporation, EA pharma Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Nioppon Kayaku Co. Ltd., Kissei Pharmaceutical Co. Ltd. Commercial research funding: AbbVie GK, Mitsubishi Tanabe Pharma Corporation, EA pharma Co. Ltd., Mochida Pharmaceutical Co., Ltd., Nioppon Kayaku Co. Ltd., JIMRO Co. M. NAGAHORI. Honoraria: Kissei Pharmaceutical Co. Ltd., WithdrawalTakeda Pharmaceutical Co. Ltd., Kyorin ofPharmaceutical thiopurines Co. Ltd., Mochida Pharmaceutical Co.,in Ltd., AbbVieCrohn’s GK, Mitsubishi Tanabe Pharmadisease Corporation, Nioppon Kayaku Co. -

Weekend Special
THE NEST WEEKEND SPECIAL JUNE 6 & 7, 2020 Member Standard JUNE 13 & 14, 2020 Member Standard BRAISED KAMPUNG 22.5 24.5 ‘KUNG PAO’ PRAWN 22.5 24.5 SPRING CHICKEN (HALF) Sautéed Prawn with Dried Chilli, Onion, Ginger, Spring Marinated with Dark Soy Sauce, Japanese Shoyu and Onion, Tri-Colour Capsicum Garnished with Cashew Nuts Ginger, Slight Deep-Fried then Braised with Garlic, Shallot, Ginger, Leek, Superior Oyster Sauce and Ginseng Roots LEMON CHICKEN CUTLET 15.5 16.5 Deep-Fried Boneless Chicken Thigh Coated with OATMEAL SEABASS 22.5 24.5 Breadcrumbs Accompanied with Homemade Lemon Sauce Crispy Seabass Fillet Stir-Fried with Oatmeal, Curry Leaves, Chilli Padi and Margarine BLACK GAROUPA (WHOLE) 32.5 34.5 Deep-Fried Black Garoupa Served with Caramelized Onion SALTED EGG YOLK BITTER GOURD 12.5 13.5 and Dark Sauce Sautéed Bitter Gourd with Salted Egg Yolk All prices are in Singapore dollars and subject to prevailing government tax PLEASE CALL +65 6248 1711 FOR ORDERS SELF-COLLECT AND DELIVERIES AVAILABLE 10% OFF SELF-COLLECT ORDERS OPERATING HOURS: 11AM TO 7PM 11 LAGUNA GOLF GREEN SINGAPORE 488047 THE NEST WEEKEND SPECIAL FATHER’S DAY LUCKY DRAW PROMOTION Every $58 spent (single receipt, before GST) in June entitled one entry for our lucky draw! Prize worth more than $1,000 includes: One round of Weekday Golf at Masters Course for two persons Two $58 F&B vouchers One bottle of Lagavulin 16 Years Single Malt Scotch Whisky *Preorder required. Last order on June 18, 2020 before 3.00pm JUNE 20 & 21, 2020 FATHER’S DAY SPECIALS ADD-ONS (SERVING FOR 4 – 6 PERSONS) Member Standard (SERVING FOR 4 – 6 PERSONS) Member Standard * ROASTED DUCK – CHINESE STYLE 58 68 * ASIAN SIDE DISHES 38 48 (WHOLE) Mixed Salad Greens with Kombu Dressing, Stir-Fried Roasted Duck Served with Plum Sauce Kai Lan with Mushrooms and Chef’s Special Fried Rice * ROASTED BEEF BRISKET (APPROX. -

Japan Energy and Sports Drink Market: Sample Report
1 © This is a licensed product of Ken Research and should not be copied TABLE OF CONTENTS 1. Asia Energy and Sports Drinks Market Introduction 2. Asia Energy and Sports Drinks Market Size, 2007-2012 3. Japan Energy and Sports Drinks Market Introduction 4. Japan Energy and Sports Drinks Market Size, 2007-2012 5. Japan Energy and Sports Drinks Market Segmentation by Functionality, 2007-2012 5.1. For Consumers at Work, 2007-2017 5.2. For Consumers at Play, 2007-2017 5.3. For Consumers at Leisure, 2007-2017 6. Japan Quasi Drug Energy Drink Market Segmentation by Distribution Channel, 2007-2012 6.1. For Drug Stores, 2007-2017 6.2. For Convenience Stores, 2007-2017 6.3. For Supermarkets and Hypermarkets, 2007-2017 6.4. For Others (Small Independent Retailers and Conventional Grocery Stores), 2007-2017 7. Japan Energy Drink Market Segmentation by Distribution Channel, 2007-2012 7.1. For Vending Machines, 2007-2017 7.2. For Convenience Stores, 2007-2017 7.3. For Supermarkets and Hypermarkets, 2007-2017 7.4. For Others (Small Independent Shops and Retailers), 2007-2017 8. Japan Sports Drink Market Segmentation by Distribution Channel, 2007-2012 8.1. For Hypermarkets and Supermarkets, 2007-2017 8.2. For Convenience Stores, 2007-2017 8.3. For Vending Machines, 2007-2017 8.4. For Others (Small Independent Retailers and Grocery Stores), 2007-2017 9. Japan Energy and Sports Drinks Market Trends and Developments Surge in the Number of Vending Machines in Japan Increasing Number of Fitness Clubs and Programs 2 © This is a licensed product of Ken Research and should not be copied Increase in the Expenditure on Food and Beverage Products 10. -

John E. Flaherty Ravin R. Patel MCCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102 T (973) 622-4444 F (973) 624-7070
Case 3:15-cv-07635-MLC-TJB Document 37 Filed 06/01/16 Page 1 of 13 PageID: 300 John E. Flaherty Ravin R. Patel MCCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102 T (973) 622-4444 F (973) 624-7070 Attorneys for Plaintiffs Of Counsel: Eric J. Lobenfeld Arlene L. Chow HOGAN LOVELLS US LLP 875 Third Avenue New York, New York 10022 T (212) 918-3000 F (212) 918-3100 Attorneys for Plaintiffs IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF NEW JERSEY : TAKEDA PHARMACEUTICAL COMPANY : LIMITED, TAKEDA PHARMACEUTICALS : Civil Action No. 3:15-cv-07635-MLC-TJB U.S.A., INC., and TAKEDA : PHARMACEUTICALS AMERICA, INC., : : AMENDED COMPLAINT FOR PATENT Plaintiffs, : INFRINGEMENT : v. : : AUROBINDO PHARMA LTD., : AUROBINDO PHARMA U.S.A., INC., and : AUROLIFE PHARMA LLC, : : Defendants. : Case 3:15-cv-07635-MLC-TJB Document 37 Filed 06/01/16 Page 2 of 13 PageID: 301 Plaintiffs Takeda Pharmaceutical Company Limited, Takeda Pharmaceuticals U.S.A., Inc., and Takeda Pharmaceuticals America, Inc. (collectively, "Plaintiffs" or "Takeda"), for their Complaint against Defendants Aurobindo Pharma Ltd., Aurobindo Pharma U.S.A., Inc., and Aurolife Pharma LLC (collectively, "Defendants" or "Aurobindo"), allege as follows: THE PARTIES 1. Plaintiff Takeda Pharmaceutical Company Limited ("Takeda Japan") is a Japanese corporation, having a principal place of business at 1-1, Doshomachi 4-chome, Chuo- ku, Osaka, Japan. As part of its business, Takeda Japan is involved in the research, development, and marketing of pharmaceutical products. Takeda Japan manufactures lansoprazole orally disintegrating tablets. 2. Plaintiff Takeda Japan is the owner of record and assignee of U.S. -

Cases and Opinions on Clinical Trials by Japanese Pharmaceutical Companies in China
Cases and Opinions on Clinical Trials by Japanese Pharmaceutical Companies in China Man Shii Otsuka Beijing Research Institute March 22, 2012 Development Status of Japanese Pharmaceutical Companies in China Cases on Clinical Trials by OBRI Opinions on Development Status in China Development Status of Japanese Pharmaceutical Companies in China Merit for Conducting MCTs in Japan and China Chinese and Japanese are very similar in genes Nature 437: 1299-1320 (2005) 4 Japanese Basic Principles on Global Clinical Trials Japanese version English version Japanese:http://www.pmda.go.jp/operations/notice/2007/file/0928010.pdf English :http://www.pmda.go.jp/operations/notice/2007/file/0928010-e.pdf 5 Japanese Pharmaceutical Companies in China (China local corporation) 1 Eisai China Inc 18 China Otsuka Pharmaceutical Co., Ltd Kirin Kunpeng (China) Bio-Phramaceutical Otsuka Pharmaceutical Group 2 19 Co.,LTD Zhejiang Otsuka Pharmaceutical Co., Ltd Takeda Pharmaceutical (China) Co., Ltd. 3 20 Guang Dong Otsuka Pharmaceutical Co., Ltd Tianjin Takeda Pharmaceutical Company 4 Limited 21 Sichuan Otsuka Pharmaceutical Co., Ltd Sumitomo Pharmaceutical ( Suzhou) Company 5 Limited 22 Suzhou Otsuka Pharmaceutical Co., Ltd Asahi Kasei Management ( Shanghai) Company 6 Limited 23 Mitsubishi Pharma Research & Development(BeiJing) Kowa(Shanghai) pharmaceutical consulting 7 Co.,Ltd 24 Mitsubishi Pharma Research & Development(GuangZhou) Santen Pharmaceutical Co., Ltd Kyowa Hakko Pharmaceutical 8 25 Technology (Shanghai)Co.,Ltd Chugai Pharmal(ShangHai)Consulting Co., 9 Ltd. 26 Shanghai Tsumura Pharmaceutical. Co.,Ltd 10 CHUGAI Pharmaceutical Beijing Co., Ltd 27 Tianjin ROHTO Herbal Medicine Co., Ltd 11 Teijin Pharma Shanghai Consulting Co.,LTD 28 Nitto denko (Shanghai) pharmaceutical consulting Limited Senju Pharmaceutical 12 29 Shanghai Ajinomoto amino acid limited company Science & Technology(BeiJing)Co.,LTD 13 Taiho Pharmaceutical of Beijing Co.,Ltd. -

17Th DIA Japan Annual Meeting 2020 -Beyond Innovation
17th DIA Japan Annual Meeting 2020 -Beyond Innovation- November 8-10, 2020 DIAglobal.org/Japan2020 PROGRAM CHAIR Yuji Kumagai, MD, PhD Program Overview Kitasato University Hospital PROGRAM VICE-CHAIR Amidst the backdrop of the fourth industrial revolution, recent years have seen the launch of Keiichi Inaizumi, MSc initiatives related to various innovations that will create new value and bring about major changes Pfizer R&D Japan for our society. These innovations are an outcome of the creation and application of new techniques and technologies exemplified by the use of artificial intelligence, big data, and medical and genomic PROGRAM COMMITTEE information in various fields, including research and development and lifecycle management of Taro Amagasaki, PhD medical devices. Novartis Pharma K.K. Currently, the global COVID-19 pandemic has reaffirmed the importance of the role played by Nobuyuki Hanamura, PhD, MBA IQVIA Services Japan K.K. medical products in providing treatment and medications to support the health and safety of Toshiko Ishibashi, PhD, RN people everywhere. At the same time however, efforts to take on new challenges aimed at creating Ono Pharmaceutical Co., Ltd. new innovations that break away from conventional wisdom are underway around the globe. Kazuhiko Ishida, MS, RPh Astellas Pharma Inc. As society adapts to the ongoing presence of COVID-19, it is becoming more and more important Yayoi Kitawaki, MS that we actively seek to learn the latest technologies and initiatives that will underpin various Novartis Pharma K.K. innovations, consider how our jobs will change and how we should reimagine those jobs moving Yoshiko Komuro, PhD forward, and develop the skills and mindset that will allow us to respond and adapt to these major Pharmaceuticals and Medical Devices changes.