Article the Health Impact Fund
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Journal of Diabetes in Developing Countries Incorporating Diabetes Bulletin
International Journal of Diabetes in Developing Countries Incorporating Diabetes Bulletin Founder Editors Late M. M. S. Ahuja Hemraj B. Chandalia, Department of Endocrinology, Diabetes, Metabolism, Jaslok Hospital and Research Center, Mumbai Editor-in-Chief S.V. Madhu, Department of Endocrinology, University College of Medical Sciences-GTB Hospital, Delhi Executive Editor Rajeev Chawla, North Delhi Diabetes Centre, Delhi Associate Editors Amitesh Aggarwal, Department of Medicine, University College of Medical Sciences & GTB Hospital, Delhi, India Sudhir Bhandari, Department of Medicine, SMS Medical College and Hospital, Jaipur, India Simmi Dube, Department of Medicine, Gandhi Medical College & Hamidia Hospital Bhopal, MP, India Sujoy Ghosh, Department of Endocrinology, Institute of Post Graduate Medical Education and Research, Kolkata, India Arvind Gupta, Department of Internal Medicine and Diabetes, Jaipur Diabetes Research Centre, Jaipur, India Sunil Gupta, Sunil’s Diabetes Care n' Research Centre Pvt. Ltd., Nagpur, India Viswanathan Mohan, Madras Diabetes Research Foundation, Chennai, India Krishna G. Seshadri, Sri Balaji Vidyapeeth, Chennai, India Saurabh Srivastava, Department of Medicine, Government Institute of Medical Sciences, Greater Noida, India Vijay Viswanathan, MV Hospital for Diabetes and Prof M Viswanthan Diabetes Research Centre Chennai, India Statistical Editors Amir Maroof Khan, Community Medicine, University College of Medical Sciences and GTB Hospital, Delhi Dhananjay Raje, CSTAT Royal Statistical Society, London, -

Form IEPF-1 2010-2011 FINAL
Read the following instructions carefully before proceeding to enter the Investor Details: (i)Install the pre-requisite softwares to proceed. The path for the same is as follows: MCA Portal >> Investor Services >> IEPF >> IEPF Application >> Prerequisite Software (ii) Upload the excel file in the format xls only Important Note (iii) Do not make any changes in the excel format/sheet name. Also do not delete any tab/sheet in the file PLEASE NOTE: 1) Kindly ensure that Summation of amounts in the excel(s) should be equal to that in IEPF Form, else your excel shall get rejected. 2) Kindly ensure that AGM Date in the excel(s) should be same as in IEPF Form, else your excel shall get rejected. Steps to follow to fill details in the 'Investor Details' tab. It is important that you Enable Macro using following instructions : Enable Macros a) Excel 2000 and 2003: Tools-->Macro-->Security-->Select 'Low'-->OK b) Excel 2007: Office Button-->Excel Options-->Trust Center-->Trust Center Settings--Macro Settings-->Enable all Macros-->OK Pl Note : Close the Excel Sheet and re-open it after enabling Macro to start. Enter the CIN (i) Enter the CIN in the excel (cell B2) (ii) Click on "Prefill" button (iii) Company Name will be automatically filled on click of prefill button . User need not enter it. (i) Fill in the required details in Columns A to P for Investor Details (row 15 onwards) (ii) Follow the below mentioned validations for filling in the details. First Name -> Mandatory if 'Last Name' is blank and Length should be less or equal to 35 characters. -

List of Common Service Centres Established in Uttar Pradesh
LIST OF COMMON SERVICE CENTRES ESTABLISHED IN UTTAR PRADESH S.No. VLE Name Contact Number Village Block District SCA 1 Aram singh 9458468112 Fathehabad Fathehabad Agra Vayam Tech. 2 Shiv Shankar Sharma 9528570704 Pentikhera Fathehabad Agra Vayam Tech. 3 Rajesh Singh 9058541589 Bhikanpur (Sarangpur) Fatehabad Agra Vayam Tech. 4 Ravindra Kumar Sharma 9758227711 Jarari (Rasoolpur) Fatehabad Agra Vayam Tech. 5 Satendra 9759965038 Bijoli Bah Agra Vayam Tech. 6 Mahesh Kumar 9412414296 Bara Khurd Akrabad Aligarh Vayam Tech. 7 Mohit Kumar Sharma 9410692572 Pali Mukimpur Bijoli Aligarh Vayam Tech. 8 Rakesh Kumur 9917177296 Pilkhunu Bijoli Aligarh Vayam Tech. 9 Vijay Pal Singh 9410256553 Quarsi Lodha Aligarh Vayam Tech. 10 Prasann Kumar 9759979754 Jirauli Dhoomsingh Atruli Aligarh Vayam Tech. 11 Rajkumar 9758978036 Kaliyanpur Rani Atruli Aligarh Vayam Tech. 12 Ravisankar 8006529997 Nagar Atruli Aligarh Vayam Tech. 13 Ajitendra Vijay 9917273495 Mahamudpur Jamalpur Dhanipur Aligarh Vayam Tech. 14 Divya Sharma 7830346821 Bankner Khair Aligarh Vayam Tech. 15 Ajay Pal Singh 9012148987 Kandli Iglas Aligarh Vayam Tech. 16 Puneet Agrawal 8410104219 Chota Jawan Jawan Aligarh Vayam Tech. 17 Upendra Singh 9568154697 Nagla Lochan Bijoli Aligarh Vayam Tech. 18 VIKAS 9719632620 CHAK VEERUMPUR JEWAR G.B.Nagar Vayam Tech. 19 MUSARRAT ALI 9015072930 JARCHA DADRI G.B.Nagar Vayam Tech. 20 SATYA BHAN SINGH 9818498799 KHATANA DADRI G.B.Nagar Vayam Tech. 21 SATYVIR SINGH 8979997811 NAGLA NAINSUKH DADRI G.B.Nagar Vayam Tech. 22 VIKRAM SINGH 9015758386 AKILPUR JAGER DADRI G.B.Nagar Vayam Tech. 23 Pushpendra Kumar 9412845804 Mohmadpur Jadon Dankaur G.B.Nagar Vayam Tech. 24 Sandeep Tyagi 9810206799 Chhaprola Bisrakh G.B.Nagar Vayam Tech. -

Toward a Social History of Beadmakers
BEADS: Journal of the Society of Bead Researchers Volume 6 Volume 6 (1994) Article 7 1-1-1994 Toward a Social History of Beadmakers Peter Francis Jr. Follow this and additional works at: https://surface.syr.edu/beads Part of the Archaeological Anthropology Commons, History of Art, Architecture, and Archaeology Commons, Science and Technology Studies Commons, and the Social and Cultural Anthropology Commons Repository Citation Francis, Peter Jr. (1994). "Toward a Social History of Beadmakers." BEADS: Journal of the Society of Bead Researchers 6: 61-80. Available at: https://surface.syr.edu/beads/vol6/iss1/7 This Article is brought to you for free and open access by SURFACE. It has been accepted for inclusion in BEADS: Journal of the Society of Bead Researchers by an authorized editor of SURFACE. For more information, please contact [email protected]. TOW ARD A SOCIAL HISTORY OF BEAD MAKERS Peter Francis, Jr. An understanding of beads requires an understanding of the w.ith the promise of special privileges or people involved with them. This paper examines three his pecuniary gain. torical aspects ofpeople engaged in beadmaking, especially Whether the movement is because of a "push" the production of glass beads. The history of their social or a "pull," it is easy for glass beadmakers to do. relations is considered in regards to the record of their They are not peasants tied to the land. Their chief physical movements, the manner in which they organize themselves and pass on their traditions, and their status raw materials are widespread, and special within society. Information concerning each of these is ar materials-such as colorants-have long been ranged geographically and chronologically in an attempt to articles of commerce. -

GIPE-208819-Contents.Pdf (10.25Mb)
THE BOOK At the Census of India, in 1881, an attempt was lll3de to obtain the materials for a complete list of all Castes and Tribes as 1 eturned by the. people themselves .and ente red by the Census Enumerators in their Schedutes. Instructions were sent to .each Province and Native State directing that the number of each Caste recorded, .and the composition of each Caste by sex should be shown in the .:final report. In this manner it was designed to lay "a foundation for further research into the little l-nown subject of Caste," a subject .in inquiring into which investigators have been gravelled, not for lack of matter but from its abundance and complexity, and the lack of all rational arran~ment. The subject as a whole has indeed been a mighty maze without a plan. An inquirer .into the social habits and customs of a Caste in nne district h.a.s always been liable to the .subse quent dis.covery that the people whom he had met were but offshoots or wanderers from a larger Tribe whose home was in another province. The distinctive habits and customs of a people are of course always freshest and most marked where the mass of that people dwell : and when a detachment wanders away or splits off from the parent Tribe and settles elsewhere, it suffers, notwithstanding its Caste-conserv ancy. a certain change through the moul ding influence of superior numbers around. Hence the desideratum of a bird'.s-eye view of the entire system of Castes and Tribes found in India : and this, as far as tlteir strength and distribution go, is what 1 have tried to supply in this Compendium. -

Ethnography : Castes and Tribes
िव�ा �सारक मंडळ, ठाणे Title : Ethnography : Caste and Tribes Author : Baines, Athelstane Publisher : Strassburg : Verlang Von Karl J. Trubner Publication Year : 1912 Pages : 231 pgs. गणपुस्त �व�ा �सारत मंडळाच्ा “�ंथाल्” �तल्पा्गर् िनिमर्त गणपुस्क िन�म्ी वषर : 2014 गणपुस्क �मांक : 101 ^ k^ Grundriss der Indo-arischen Philologie und Altertumskunde (ENCYCLOPEDIA OF INDO-ARYAN RESEARCH) BEGRUNDET VON G. BUHLER, FORTGESETZT VON F. KIELHORN, HERAUSGEGEBEN VON H. LUDERS UND J. WACKERNAGEL. II. BAND, 5. HEFT. ETHNOGRAPHY (CASTES AND TRIBES) BY SIR ATHELSTANE BAINES WITH A LIST OF THE MORE IMPORTANT WORKS ON INDIAN ETHNOGRAPHY BY W. SIEGLING. ^35^- STRASSBURG VERLAG VON KARL J. TRUBNER 1912. M. DuMout Schauberg, StraCburg. 6RUNDRISS DER INDO-ARISCHEN PHILOLOGIE UND ALTERTUMSKUNDE (ENCYCLOPEDIA OF INDO-ARYAN RESEARCH) BEGRiJNDET VON G. BUHLER, FORTGESETZT VON F. KIELHORN, HERAUSGEGEBEN VON H. LUDERS UND J. WACKERNAGEL. II. BAND, 5. HEFT. ETHNOGRAPHY (CASTES AND TRIBES) BY SIR ATHELSTANE BAINES. INTRODUCTION. § I. The subject with which it is proposed to deal in the present work is that branch of Indian ethnography which is concerned with the social organisation of the population, or the dispersal of the latter into definite groups based upon considerations of race, tribe, blood or oc- cupation. In the main, it takes the form of a descriptive survey of the return of castes and tribes obtained through the Census of 1901. The scope of the review, however, is limited to the population of India properly so called, and does not, therefore, include Burma or the outlying tracts of Baliichistan, Aden and the Andamans, by the omission of which the population dealt with is reduced from 294 to 283 millions. -

Social Transformation of Pakistan Under Kashmir Dispute
Social Transformations in Contemporary Society, 2019 (7) ISSN 2345-0126 (online) SOCIAL TRANSFORMATION OF PAKISTAN UNDER KASHMIR DISPUTE Sohaib Mukhtar National University of Malaysia, Malaysia [email protected] Abstract Kashmir dispute is the most important issue between India and Pakistan as they have fought three major wars and two conflicts since 1947. Kashmir dispute arose when British India was separated into Pakistan and India on 15th August 1947 under Indian Independence Act 1947. Independent Indian States could accede either to Pakistan or India as on 26th October 1947, Hari Singh signed treaty of accession with Indian Government while the Governor General of India Mountbatten remarked that after clearance from insurgency, plebiscite would take place in the state and people of Kashmir would decide either to go with Pakistan or India. During war of Kashmir in 1947, India went to the United Nations (UN) and asked for mediation. The UN passed resolution on 20th January 1948 to assist peaceful resolution of Kashmir dispute as another resolution was passed on 21st April 1948 for organization of plebiscite in Kashmir. India holds 43% of the region, Pakistan holds 37% and remaining 19% area is controlled by China. Dispute of Kashmir is required to be resolved through mediation under UN resolutions. Purpose – This research is an analysis of Kashmir dispute under the light of historical perspective, law passed by British Parliament and UN resolutions to clarify Kashmir dispute and recommend its solution under the light of UN resolutions. Design/methodology/approach – This study is routed in qualitative method of research to analyze Kashmir dispute under the light of relevant laws passed by British Parliament, historical perspective, and resolutions passed by the UN. -

Redacted Thesis File
Context for improving access to care for children and youth with diabetes in less- resourced countries. Graham David OGLE ORCID 0000-0002-2022-0866 Doctor of Medical Science June 2020 Faculty of Medicine, Dentistry and Health Sciences Submitted in total fulfilment of the requirements for the degree of Doctor of Medical Science at the University of Melbourne by compilation of published papers. 1 1.0 THESIS ABSTRACT There are major deficits in knowledge related to the epidemiology and care of the various types of diabetes in young people in less-resourced countries. Multiple barriers exist at individual, community, health system, national, and international levels that must be overcome to lessen the gap in outcomes between advantaged and disadvantaged regions. This thesis presents 11 published papers by the candidate addressing this gap in knowledge. Type 1 diabetes (T1D) incidence data is presented for three countries with no previous data (Fiji, Bolivia and Azerbaijan), showing differing rates in each country, and in Fiji differing rates in the two main ethnic populations. Novel information on the types of diabetes is presented for Azerbaijan (along with the incidence data aforementioned), Pakistan and Bangladesh. Results in Azerbaijan were similar to those seen in European populations. In Pakistan and Bangladesh, it is common to see atypical forms that clinically present like T1D cases but do not have low C-peptide values or diabetes autoantibodies. Five papers examine costs and access to care. In a survey of 71 countries, availability of nearly all key components of care was greatly reduced in lower-income countries. A study of costs to families in 15 countries demonstrated that the cost of core supplies is prohibitively expensive for many families. -

Supreme Court of India Miscellaneous Matters to Be Listed on 23-03-2020
SUPREME COURT OF INDIA MISCELLANEOUS MATTERS TO BE LISTED ON 23-03-2020 ADVANCE LIST - AL/50/2020 SNo. Case No. Petitioner / Respondent Petitioner/Respondent Advocate 1 SLP(C) No. 15053/2011 SHANKARAPPA AND ORS. V. N. RAGHUPATHY IV-A Versus SRI YAMANAPPA (D) BY LRS. SMT. DUNDAWWA AND ORS. ANKUR S. KULKARNI 2 SLP(Crl) No. 8842/2011 UNION OF INDIA B. KRISHNA PRASAD II-A Versus MURAKONDA VENKAT NARAYANA AMOL B. KARANDE[R-1] IA No. 21324/2011 - CONDONATION OF DELAY IN FILING IA No. 21325/2011 - CONDONATION OF DELAY IN REFILING / CURING THE DEFECTS IA No. 22774/2011 - EXEMPTION FROM FILING C/C OF THE IMPUGNED JUDGMENT IA No. 22773/2011 - STAY APPLICATION 3 C.A. No. 8517/2011 U.P. POWER CORPORATION LTD.. AND ANR. RAKESH UTTAMCHANDRA III-A UPADHYAY Versus M/S. JAGANNATH STEELS PVT. LTD. ALOK SHUKLA IA No. 35630/2020 - EXEMPTION FROM FILING O.T. IA No. 35628/2020 - STAY APPLICATION 4 C.A. No. 2207/2012 M/S VENUS RAMEDIES LTD. MOHAN LAL SHARMA IV Versus COMMISSIONER OF CENTRAL EXCISE B. KRISHNA PRASAD IA No. 23858/2020 - WITHDRAWAL OF CASE / APPLICATION 5 SLP(C) No. ARABIAN EXPORTS PRIVATE LTD. K J JOHN AND CO 16907-16908/2012 IX Versus NATIONAL INSURANCE CO. LTD. MANJEET CHAWLA 6 Crl.A. No. 1317/2012 SIVASANKAR VIJAY KUMAR (SCLSC) II-C Versus THE STATE OF TAMIL NADU V. G. PRAGASAM I.A.No.32246 of 2020 i.e application for bail is to be listed only. IA No. 32246/2020 - GRANT OF BAIL 7 SLP(C) No. -

SIS-NL-1933-16-48.Pdf (1.029Mb)
,Tbe S~r"ant of India EnrTOll.: P. KODANDA RAO. OrneK: SERVA.NT!! 011 INDIA. SootBn's' HOME, POONA. 4. POONA-'rHURSDAY, DECEMBER 7,.1933; { lNDIA.N SUBSN RsA' V QL. XV1,. 1lT0. 48,} FORIIIIGN • 15s.' OONTENTS, oppositiOD in any quarter. The proposed ....8IlgetIillllt oannot of oourse satisfy that section oi-opinion whieb wanted Beraw to join tha fedsrBtion as a iull.floogwd ~O._W'ER, Indian province; but it Dan oonsole itself witb tb.& AJlftOLBB _ refleotion-and surely - if; ie no negligible glllin -that the worst fears of the puhlio about th& Indian CUrr.... :r and.Ezoh8llge. , ... 567 569 tran.fer to the Nizam of BeraT's political destiniea SUrer Agreement. by a· stroke of th& pen Imd behind its baok have not 'rho Buorn Bank BUL. By come true. !'ref. D. G.. XKrn, M. A. But the point of more immediete oon08rn to the on 1.0800" LBTTER. people of Berar relates to the status of ita representat atives in the fedsral legislatures.. If Berar is to' be BBvucw:- reg!lllded as owning allegiance to the Nimam its Probl... o£plg_SPrejudloe. By X.1I8DBka· ••• S?4 representatives like· those hailing from other State ratJla1Dlr tenitories will have· to he the nominees of' the '111ler SHOH l'IOftOlt. in thie osse the Nizam. How would Bm'arie'relisb the idea of their repr8l!8ntatives sitting in Ihe federal (loJlUSPOMDUOB :- legislature not in virtue of their election .by their "1uam LegillatloD aDd Soola\J'UI"ae~ ,. oompeers bu~ 11& tbe Nizam's nominees? It is true 117 :1'. R. -

Concept and Evolution of Dowry *Soumi Chatterjee Research Scholar, School of Law and Legal Affairs, Noida International University, Greater Noida
International Journal of Humanities and Social Science Invention (IJHSSI) ISSN (Online): 2319 – 7722, ISSN (Print): 2319 – 7714 www.ijhssi.org ||Volume 7 Issue 01||January. 2018 || PP.85-90 Concept and Evolution of Dowry *Soumi Chatterjee Research Scholar, School of Law and Legal Affairs, Noida International University, Greater Noida. Corresponding Author:*Soumi Chatterjee Abstract: The concept of dowry has been around since many centuries and led to many told and untold stories of crime, cruelty and harassment of brides during or after marriage. This article discusses the evolution of the concept of dowry in India and also few other countries and the laws prohibiting the same in India. Dowry means giving of wealth or property to the Groom or his family by the Bride‘s family at the time of their marriage. The Dowry Prohibition Act, 1961 makes the giving and taking of dowry void and illegal. Section 498A IPC penalises the husband and his family members in case there is an act of cruelty on the bride within seven years of marriage. The section makes the crime non bailable and non-compoundable. These laws are absolutely pro- women and requires very little prior corroboration in case there is any complaints under these provisions of law and this has given certain section of women the freedom to misuse these sections to fulfil their mala-fide motives. Key Words:Dowry, Misuse, Anti-Dowry Laws. ----------------------------------------------------------------------------------------------------------------------- ---------------- Date of Submission: 21-01-2018 Date of acceptance: 05-02-2018 ----------------------------------------------------------------------------------------------------------------------- ---------------- I. Introduction ―Man and Women are both equal and play vital roles in the creation and development of their families in particular and society in general.‖1 ---J KrishnaMurthy India is a vast and multi-cultural country. -
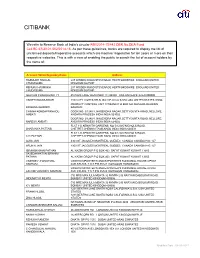
Details of Unclaimed Deposits / Inoperative Accounts
CITIBANK We refer to Reserve Bank of India’s circular RBI/2014-15/442 DBR.No.DEA Fund Cell.BC.67/30.01.002/2014-15. As per these guidelines, banks are required to display the list of unclaimed deposits/inoperative accounts which are inactive/ inoperative for ten years or more on their respective websites. This is with a view of enabling the public to search the list of account holders by the name of: Account Holder/Signatory Name Address HASMUKH HARILAL 207 WISDEN ROAD STEVENAGE HERTFORDSHIRE ENGLAND UNITED CHUDASAMA KINGDOM SG15NP RENUKA HASMUKH 207 WISDEN ROAD STEVENAGE HERTFORDSHIRE ENGLAND UNITED CHUDASAMA KINGDOM SG15NP GAUTAM CHAKRAVARTTY 45 PARK LANE WESTPORT CT.06880 USA UNITED STATES 000000 VAIDEHI MAJMUNDAR 1109 CITY LIGHTS DR ALISO VIEJO CA 92656 USA UNITED STATES 92656 PRODUCT CONTROL UNIT CITIBANK P O BOX 548 MANAMA BAHRAIN KRISHNA GUDDETI BAHRAIN CHINNA KONDAPPANAIDU DOOR NO: 3/1240/1, NAGENDRA NAGAR,SETTYGUNTA ROAD, NELLORE, AMBATI ANDHRA PRADESH INDIA INDIA 524002 DOOR NO: 3/1240/1, NAGENDRA NAGAR,SETTYGUNTA ROAD, NELLORE, NARESH AMBATI ANDHRA PRADESH INDIA INDIA 524002 FLAT 1-D,KENWITH GARDENS, NO 5/12,MC'NICHOLS ROAD, SHARANYA PATTABI CHETPET,CHENNAI TAMILNADU INDIA INDIA 600031 FLAT 1-D,KENWITH GARDENS, NO 5/12,MC'NICHOLS ROAD, C D PATTABI . CHETPET,CHENNAI TAMILNADU INDIA INDIA 600031 ALKA JAIN 4303 ST.JACQUES MONTREAL QUEBEC CANADA CANADA H4C 1J7 ARUN K JAIN 4303 ST.JACQUES MONTREAL QUEBEC CANADA CANADA H4C 1J7 IBRAHIM KHAN PATHAN AL KAZMI GROUP P O BOX 403 SAFAT KUWAIT KUWAIT 13005 SAJEDAKHATUN IBRAHIM PATHAN AL KAZMI