Influence of the Ph of Chardonnay Must on Malolactic Fermentation In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malolactic Fermentation in Red Wine
Fact Sheet WINEMAKING Malolactic fermentation in red wine Introduction Malolactic fermentation (MLF) is a secondary bacterial fermentation carried out in most red wines. Oenococcus oeni, a member of the lactic acid bacteria (LAB) family, is the main bacterium responsible for conducting MLF, due to its ability to survive the harsh conditions of wine (high alcohol, low pH and low nutrients) and its production of desirable wine sensory attributes. One of the important roles of MLF is to confer microbiological stability towards further metabolism of L-malic acid. Specifically, MLF removes the L-malic acid in wine that can be a carbon source for yeast and bacterial growth, potentially leading to spoilage, spritz and unwanted flavours. MLF can also be conducted in some wines to influence wine style. MLF often occurs naturally after the completion of primary fermentation or can also be induced by inoculation with a selected bacterial strain. Since natural or ‘wild’ MLF can be unpredictable in both time of onset and impact on wine quality, malolactic starter cultures are commonly used. In Australia, almost all red wines undergo MLF and 74% are inoculated with bacterial starter cultures (Nordestgaard 2019). This fact sheet provides practical information for induction of MLF in red wine. A separate fact sheet provides equivalent information for white and sparkling base wine. These should also be read in conjunction with another fact sheet, Achieving successful malolactic fermentation, which gives further practical guidelines for MLF induction, monitoring and management. Updated September 2020 Fact Sheet WINEMAKING Key parameters for a successful MLF in red wine Composition of red wine/must The main wine compositional factors that determine the success of MLF are alcohol, pH, temperature and sulfur dioxide (SO2) concentration. -
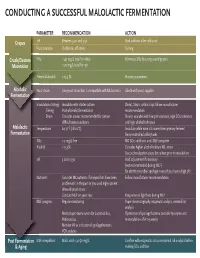
Conducting a Successful Malolactic Fermentation
CONDUCTING A SUCCESSFUL MALOLACTIC FERMENTATION PARAMETERPA RECOMMENDATION ACTION Grapes pH Between 3.20 and 3.50 Acid addition after cold soak FruitFru condition Visible rot, off odors Sorting Crush/Destem SO2SO < 40 mg/L total for white Minimize SO2 by using sound grapes Maceration < 70 mg/L total for red PotentialPPot alcohol < 13.5 % Harvest parameters Alcoholic YeastYeY a strain Use yeast strain that is compatible with ML bacteria Check with yeast supplier Fermentation InoculationIno strategy Inoculate with starter culture Direct, Step 1, or Build up: follow manufacturer Timing Post-alcoholic fermentation recommendation Strain Consider strains recommended for certain Strains available with low pH tolerance, high SO2 tolerance difficult wine conditions and high alcohol tolerance Malolactic TemperatureTem 64-71°F (18-22°C) Inoculate while wine still warm from primary ferment Fermentation Temp-controlled cellar/tanks SO2SO < 5 mg/L free NO SO2 additions until MLF complete AlcoholAlc < 13.5% Consider higher alcohol tolerant ML strain Use acclimatization steps for culture prior to inoculation pH 3.20 to 3.50 Acid adjustment if necessary (not recommended during MLF) Be alert to microbial spoilage issues if you have a high pH NNutrientsu Consider ML nutrients if vineyard lots have been Follow manufacturer recommendation problematic in the past or you used high nutrient demand yeast strain Conduct MLF on yeast lees Keep wine on light lees during MLF MMLFL progress Regular monitoring Paper chromatography, enzymatic analysis, external lab analysis Microscopic examination for Lactobacillus, If presence of spoilage bacteria consider lysozyme and Pediococcus re-inoculation after 2-3 weeks Monitor VA as indicator of spoilage bacteria PCR analysis Post Fermentation MLFML completion Malic acid < 30-50 mg/L Confirm with enzymatic assay or external lab analysis before & Aging making SO2 addition. -

Château Simone
Château Simone This historic estate, situated in the hills just south of Aix-en-Provence, has been in the hands of four generations of the Rougier family since 1830 and holds a virtual monopoly on the appellation of Palette. The property totals 120 hectares, with 28 under vine, of which 23 qualify for the Palette AOC. Its vineyards sit on limestone soils at elevations between 500 and 750 feet on the slopes of the Montaiguet, whose special microclimate is influenced by the encircling pine forests, the mass of the Mont Sainte-Victoire, and the Arc River. The vineyards were reconstituted after phylloxera and many vines are over a century old. In an industry that moves quicker than ever and presents ever-increasing novelty, an estate like Simone is an anchor of meaning and a lens into Provence's patrimony. Viticulture: • Farming: Ecocert Organic Certification Pending. Viticulture has always been practicing organic. • Treatments : No herbicides, chemical fertilizers, or synthetic treatments • Ploughing: Extensive ploughing and working of the soil by tractor. • Soils: Limestone Scree • Vines: Head-trained vines, many over 100-years old, that are replanted on a vine-by-vine basis to maintain a healthy vineyard • Yields: Old vines and extensive debudding lower yields and eliminate the need for a green harvest. • Harvest: Entirely manual harvest, grapes sorted in the vineyard and sorted again in the cellar • Purchasing: Always entirely estate fruit Vinification: Aging: • Fermentation: All wines are fermented spontaneously in large • Élevage: Palette wines are stored in foudres for 18 months; wooden foudres with temperature control for 2 weeks. red and white wines spend an additional year in neutral barrel. -

Practical High-Acidity Winemaking Strategies for the Midwest
PRACTICAL HIGH-ACIDITY WINEMAKING STRATEGIES FOR THE MIDWEST DREW HORTON, ENOLOGY SPECIALIST UNIVERSITY OF MINNESOTA GRAPE BREEDING & ENOLOGY PROJECT GETTING STARTED •A BASIC UNDERSTANDING OF PH AND TOTAL ACIDITY IS INVALUABLE TO PRACTICAL WINE MAKING. PH VS. TA (TOTAL ACIDITY) • BOTH ARE A MEASURE OF THE RELATIVE STRENGTH OF ACIDITY IN WINE, BUT THEY DO NOT CORRELATE EXACTLY. • PH: THE INVERSE LOG OF HYDROGEN IONS IN A SOLUTION • PH: A LOGARITHMIC SCALE FROM 0 TO 14 • A PH OF 3.0 IS 10X HIGHER (OR “STRONGER”) THAN A PH OF 4.0. • TYPICALLY A PH BETWEEN 3.1 AND 3.6 IS DESIRABLE FOR WINE. PH VS. TA (TOTAL ACIDITY) • TOTAL ACIDITY IS AN EMPIRICAL MEASUREMENT OF ALL OF THE ACIDS IN A SOLUTION, OFTEN GIVEN IN GRAMS/LITER. • THE HIGHER THE ACIDITY, THE MORE TART A WINE WILL TASTE. • PH IS ABOUT MICROBIAL STABILITY, MOUTH FEEL, STRUCTURE, AND AGE-ABILITY OF A GIVEN WINE. • PH IS QUALITATIVE ACIDITY. TOTAL ACIDITY IS QUANTITATIVE… “… THE BEST THING FOR A VINEYARD ARE THE FOOTPRINTS OF THE WINEMAKER…” WINE IS MADE IN THE VINEYARD FIRST!!! • WINEMAKING STARTS IN THE VINEYARD. • VITICULTURAL PRACTICES AND HARVEST TIMING HAVE MAJOR EFFECTS • ACTIVE COLLABORATION BETWEEN VITICULTURIST AND WINEMAKER • VITICULTURE AND ENOLOGY ARE “TWO WINGS OF THE SAME BIRD”… • KNOWLEDGE OF ALL COLD-CLIMATE VARIETIES WILL HELP TO MAKE BALANCED WINES FROM THESE CHALLENGING VARIETIES. POST-VÉRAISON SUGAR/ACID CURVE ACIDS AND PH CHANGES •THROUGHOUT RIPENING, AS ACIDITY DECREASES, PH INCREASES… DETERMINING RIPENESS, AND WHEN TO PICK? RIPENESS IS MORE THAN A NUMBER. • AROMA AND FLAVOR • TEXTURE AND COLOR • CONDITION, OF VINEYARD, VINE, STEM, CLUSTER, BERRY, SKIN AND SEED… • OVERALL HEALTH, CROP LOAD, PEST ACTIVITY AND DISEASES • IMPENDING WEATHER AND PICKING CREW/MACHINERY AVAILABILITY USE THE LAB IN YOUR MOUTH AND NOSE. -

Malolactic Fermentation in Wine La Fermentation
MMALOLACTICALOLACTIC FFERMENTATIONERMENTATION IINN WWINEINE LLAA FFERMENTATIONERMENTATION MMALOLACTIQUEALOLACTIQUE DDUU VVININ LLAA FFERMENTACIÓNERMENTACIÓN MMALOLÁCTICAALOLÁCTICA DDELEL VVINOINO LLAA FFERMENTAZIONEERMENTAZIONE MMALOLATTICAALOLATTICA DELDEL VVINOINO BBIOLOGISCHERIOLOGISCHER SSÄUREABBAUÄUREABBAU ININ WEINWEIN AAPPEL-MELKSUURGISTINGPPEL-MELKSUURGISTING IINN WWYNYN A FFERMENTAÇÃOERMENTAÇÃO MMALOLÁCTICAALOLÁCTICA DDOO VINHOVINHO MMALOLAKTIALOLAKTICCNAˇ NA FFERMENTACIJAERMENTACIJA VINAVINA MALOLACTIC FERMENTATION IN WINE UNDERSTANDING THE SCIENCE AND THE PRACTICE Magali Bou (France) Piet Loubser (Republic of South Africa) Dr. Neil Brown (U.S.A.) Dr. Rich Morenzoni (U.S.A.) Dr. Peter Costello (Australia) Dr. Antonio Palacios (Spain) Dr. Richard Degré (Canada) Dr. Chris Powell (United Kingdom) Wilfried Dieterich (Germany) Katie Scully Specht (U.S.A.) Sigrid Gertsen-Briand (Canada) Gordon Specht (U.S.A.) Samantha Kollar (U.S.A.) Didier Theodore (France) Dr. Sibylle Krieger (Germany) Dr. Sylvie Van Zandycke (Belgium) Annamarie Kyne (U.S.A.) Scientifi c Editor: Dr. Rich Morenzoni Managing Editor: Katie Scully Specht Published by OCTOBER 2005 ii MALOLACTIC FERMENTATION IN WINE Production Coordinator: Claude Racine Copy editing: Judith Brown Designer: François Messier Printing: Les Impressions Au Point Certain research published or cited in this publication was funded in whole or in part by Lallemand Inc. DISCLAIMER Lallemand has compiled the information contained herein and, to the best of its knowledge, the information is true and -

Mastering Malolactic Fermentation – How to Manage the Nutrition of Wine
Mastering malolactic MAGALI fermentation – how to DÉLÉRIS-BOU & SIBYLLE manage the nutrition of KRIEGER-WEBER Lallemand SAS, Blagnac, wine bacteria and minimise France the effect of inhibitors Keywords: Malolactic fermentation, nutrition, inhibitors. 1. Introduction Malic acid penetrates into the cell in its anionic form to be Wine bacteria are characterised as having complex nutritional needs. decarboxylated into lactic acid in the cell’s cytoplasm. The de In this review, we will detail the wine bacteria’s requirements for carboxylation allows the consumption of an intracellular proton and carbon, and for nutrients containing nitrogen, vitamins and minerals. the expulsion of protons by lactate/H+ symporters. A proton gradient When a must or wine lacks certain nutritional elements, it can have – or “proton-motive force” – from the wine medium towards the cell a major impact on malolactic fermentation (MLF). Therefore, it is interior maintains the intracellular pH of the bacteria (about 6.0) and important to understand the nutritional needs of wine bacteria and to leads to forming energy as ATP. learn about the tools that are available to the winemaker for successful MLF. 2.2.2 Citric acid 2. Wine bacteria have complex nutritional Citric acid, an important component of grape must and wine, has a concentration that varies from 0.1 to 0.7 g/L. The degradation requirements pathway of citric acid by lactic bacteria leads to the formation of 2.1 Carbohydrate metabolism three types of compounds: acetic acid, lipids and acetoinic derivatives (acetoin, butanediol and diacetyl). The metabolism of citric acid is In wine, sugars are the primary source of energy for lactic bacteria, also a source of energy for O. -

Chardonnay Grape Guide By
Brehm Vineyards’ Chardonnay Grape Wine Guide v1.1 Copyright © 2011 by Peter Brehm. Not to be reprinted in any form. There Introduction are many ways to produce these styles of wine. Your Chardonnay Guide is for 20 liters of The methods described herein are personal, pressed and settled Chardonnay grape juice based on classic winemaking techniques that from one of three different vintages. The have been proven over decades. It is the way Chardonnay juices are from the White Peter Brehm, our winemaker, wishes to guide Salmon Vineyard in the Pacific Northwest’s you through this production of fine wine - it is Columbia Gorge AVA. Each of these vintage not the only way – Peter is still learning. juices will benefit from adjustment. Grape Juice Fermentation Chardonnay not only reflects the geographical Grape juice fermentation refers to the region and particular site where grown, it is a conversion of grape juice into wine by the canvas upon which the winemakers can freely actions of yeast reproduction. Brehm express their style and preferences. A Vineyards® provides you with your Chardonnay in the Chablis style is crisp, winemaking starting point and that is White fermented dry without malolactic Salmon Vineyard’s Chardonnay juice. fermentation. It is fresh and clean with little to no oak flavoring. A Burgundy style chardonnay Yeast are single celled organisms that, under usually is fermented in the presence of oak, anaerobic conditions (oxygen-free aged on the lees with much lees stirring environments), can use sugar as a carbon (batonnage). Chardonnay in the Burgundy source to grow and divide. -

Saccharomyces Cerevisiae in the Production of Fermented Beverages
beverages Review Saccharomyces cerevisiae in the Production of Fermented Beverages Graeme M Walker 1,* and Graham G Stewart 2 1 Abertay University, Dundee, Scotland DD1 1HG, UK 2 Heriot-Watt University, Edinburgh, Scotland EH14 4AS, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-1382-308658 Academic Editor: Edgar Chambers IV Received: 20 October 2016; Accepted: 11 November 2016; Published: 17 November 2016 Abstract: Alcoholic beverages are produced following the fermentation of sugars by yeasts, mainly (but not exclusively) strains of the species, Saccharomyces cerevisiae. The sugary starting materials may emanate from cereal starches (which require enzymatic pre-hydrolysis) in the case of beers and whiskies, sucrose-rich plants (molasses or sugar juice from sugarcane) in the case of rums, or from fruits (which do not require pre-hydrolysis) in the case of wines and brandies. In the presence of sugars, together with other essential nutrients such as amino acids, minerals and vitamins, S. cerevisiae will conduct fermentative metabolism to ethanol and carbon dioxide (as the primary fermentation metabolites) as the cells strive to make energy and regenerate the coenzyme NAD+ under anaerobic conditions. Yeasts will also produce numerous secondary metabolites which act as important beverage flavour congeners, including higher alcohols, esters, carbonyls and sulphur compounds. These are very important in dictating the final flavour and aroma characteristics of beverages such as beer and wine, but also in distilled beverages such as whisky, rum and brandy. Therefore, yeasts are of vital importance in providing the alcohol content and the sensory profiles of such beverages. This Introductory Chapter reviews, in general, the growth, physiology and metabolism of S. -

What Is Lysozyme and Why Is It Used in Winemaking?
5.13 What is lysozyme and why is it used in Winemaking G. S. Ritchie Lysozyme is an enzyme that is extracted from the egg white of hens but also exists in mammalian milk, tears and saliva. For many years, it has been used as a natural antimicrobial and antiviral in the food and pharmaceutical industries (Davidson and Zivanovic 2003). It can be used in winemaking to prevent microbial spoilage by gram positive bacteria, delay malolactic fermentation (MLF) or delay sulfur dioxide (SO2) additions after MLF is complete. It is usually effective within 1-3 days but does not provide a lasting protection in red wines. In contrast, lysozyme has been observed to remain active for up to six months in white wines (Bartowsky et al. 2004). Lysozyme cannot replace sulfur dioxide (SO2) because it will not prevent infections by other spoilage organisms such as Acetobacter or Brettanomyces. It is very important to monitor the wine for VA regularly and for the presence of other spoilage microbes. It will not affect desirable yeasts either. Lysozyme can be used in organic wines in the USA at concentrations up to 500 mg/L. In juices, must and wines, the main gram-positive bacteria are lactic acid bacteria (Pediococcus, Lactobacillus and Oenoccocus). We use Oenococcus in our wines to convert malic acid into lactic acid (MLF) to prevent this microbial action happening in an uncontrolled way at an undesirable time (e.g., after bottling), to lower the pH and or to change the flavor profile of our wine (e.g., to introduce buttery flavors). -
Malolactic Fermentation- Importance of Wine Lactic Acid Bacteria in Winemaking
LALLEMAND MALOLACTIC FERMENTATION- IMPORTANCE OF In an effort to compile the latest usable OF WINE LACTIC ACID BACTERIA IN WINEMAKING – IMPORTANCE MALOLACTIC FERMENTATION WINE LACTIC ACID BACTERIA information regarding malolactic fermen- tation, Lallemand published Malolactic IN WINEMAKING Fermentation in Wine - Understanding the Science and the Practice in 2005. This addition is an update to that publi- cation with new and relevant information. We intend it to be a compendium of both scientific and applied information of practical use to winemakers from all geo- graphic areas and wine growing regions. It is the desire and intention of the authors to supply the industry with information winemaking professionals can use in the pursuit and furtherance of their art. 2015 For the most recent information, log onto www.lallemandwine.com ISBN 978-2-9815255-0-5 ISBN 978-2-9815255-0-5 9 782981 525505 9 782981 525505 CouvImposéeBible June 1, 2015 8:29 AM 200p 0,46 Production coordinator: Claude Racine Copy editing: Judith Brown and Grant Hamilton Designer: François Messier Printing: Groupe Quadriscan Certain research published or cited in this publication was funded in whole or in part by Lallemand Inc. © 2015 Lallemand Inc. All rights reserved. No part of this book may be reproduced in any form or by any means whatsoever, whether electronic, mechanical, photocopying or record- ing, or otherwise, without the prior written permission of Lallemand Inc. Legal deposit Bibliothèque et Archives nationales du Québec 2015 Library and Archives Canada 2015 ISBN 978-2-9815255-0-5 DISCLAIMER: Lallemand has compiled the information contained herein and, to the best of its knowledge, the information is true and accurate. -

Oenococcus Oeni Wine Bacteria Selected by the Institut Français De La Vigne Et Du Vin (IFV) of Beaune. for Expressive and Well Balanced Red, White and Rosé Wines
® F Oenococcus oeni wine bacteria selected by the Institut Français de la Vigne et du Vin (IFV) of Beaune. For expressive and well balanced red, white and rosé wines. The MBRTM form of lactic acid bacteria represents a Lallemand specific process that subjects the lactic acid bacteria cells to various biophysical stresses, making them better able to withstand the rigors of direct addition to wine. The conditioned MBRTM lactic acid bacteria are robust and possess the ability to conduct reliable malolactic fermentation. 1 ² VITILACTIC® F is particularly recommended for malolactic fermentation in white and rosé wines under relatively challenging conditions (pH values from 3.2 and temperatures from 16°C) as well as for producing fruity red wines, medium to strongly structured wines. 2 ² pH tolerance ≥ 3.2 No production of biogenic amines Alcohol tolerance: up to 15% vol Bacteria cinnamoyl esterase negative: cannot produce SO2 tolerance: up to 50 mg/L total SO2 (pay attention to precursors for ethylphenol production by Brettanomyces molecular SO2 at low pH) Low volatile acidity production Temperature tolerance ≥ 16°C Suitable for co-inoculation and sequential inoculation Sensory contribution: . Diacetyl production: low to very moderate . Minimizes loss of colour during malolactic fermentation at low temperatures . Diminishes the vegetal and herbaceous character of wines and enhances varietal fruity aromas and roundness . Express the complexity of wines, with the enhancement of the length MLF kinetics - Syrah - Sequential inoculation Cabernet Franc, AOC Chinon (pH 3.8 – 13.95% of alcohol – 1.8 g/L of malic acid) 2 1,8 1,6 1,4 Malic Malic acis g/L 1,2 1 0,8 0,6 0,4 0,2 Fruity Vegetal Herby 0 0 3 6 9 14 17 20 24 29 35 38 42 Spontaneaous MLF Bacteria 3 time (days) Bacteria 1 Spontaneous MLF VITILACTIC F Vitilactic® F Bacteria 2 3 Use one sachet for right quantity of hL indicated on label. -

Guide to White Wine Making
Goal of this Manual: To make Great wine at home in your first try! It is highly recommended that this paper be read through completely before you start to make your wine. Wine-making is made up of a series of consecutive steps which build on and directly affect each other from the very beginning to the very end. In order to make the best wine possible you will need to make the best decisions possible at each of these steps, and in order to do that, you will need to have a general understanding of the overall process as a whole. Table of Contents Introduction Page 4 Chapter 1: Preparation & Preplanning Page 6 Chapter 2: Prepare the juice for fermentation Page 8 2.1) Prepare to Fill the Press: Crush and De-Stem the Grapes, or Whole Clusters 2.2) Let’s Clean the Slate – Adding SO2 During Processising 2.3) Fill the Press: Now or Later 2.4) Press the Grapes! 2.5) Pressing 2.6) Refining our Pressed Juice: Settling Out the Solids 2.7) Preemptive Fining 2.8) Test and Adjust the Juice Chapter 3: Add the Yeast and Begin Fermentation Page 25 3.1) Choose Your Yeast 3.2) Hydrate with Go-Ferm 3.3) "Co-Inoculation" (advanced technique) Chapter 4: Monitor Fermentation Page 28 4.1) Stir Daily 4.2) Yeast Nutrition During Fermentation 4.3) Fermentation Temperature 4.4) Monitoring your Sugars, Timing the End of Fermentation Chapter 5: Malolactic Fermentation (“MLF”) Page 34 5.1) Malolactic Fermentation Copyright 2009 MoreFlavor!, Inc Page | 2 5.2) Prepare and add the ML bacterial culture into the wine 5.3) Manageing the MLF 5.4) After 2-3 weeks, begin checking