Some Issues in Malolactic Fermentation Acid Reduction and Flavor Modification* by Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flavour Management by Citric Acid Negative MLF Starter Cultures Authors: Carsten Heinemeyer, 2B Fermcontrol; Ulrich Hamm DLR-RNH Bad Kreuznach, Germany; Dr
Flavour management by citric acid negative MLF starter cultures Authors: Carsten Heinemeyer, 2B FermControl; Ulrich Hamm DLR-RNH Bad Kreuznach, Germany; Dr. Jürgen Fröhlich, University of Mainz General will be completed in all fermentations. Additionally, acetic acid and a certain residual profile of the metabolic intermediates The malo-lactic fermentation (MLF) is a commonly used remain in the wine. method to convert the aggressive malic acid to lactic acid. This conversion results in a reduction of the titratable acidity, which New MLF Starter Cultures is desired mainly in red wine but also in numerous white wines. The development of a citric acid negative MLF starter culture This process will be done either by the indigenous flora of LAB gave a new opportunity to avoid diacetyl and acetic acid or by selected strains of LAB e.g. Oenococcus oeni. During the production from the citric acid degradation. The fermentation MLF Oenococcus oeni does not convert only malic acid into lactic with a citric acid negative MLF starter culture will best preserve acid, numerous amounts of aroma active by-products will also be the varietal character of the wine. produced. The best known is diacetyl, which gives buttery notes to the wine. Diacetyl will be produced during the MLF by the In a comprehensive four year study at the wine research institute conversion of the natural citric acid in the wine by Oenococcus DLR-RNH Bad Kreuznach in Rhineland-Palatinate-Germany, oeni (Jan Clair Nielsen 1999). Apart from the diacetyl formation different MLF starter strains were tested under practical by Oenococcus oeni, numerous intermediate by-products are winemaking conditions. -

Malolactic Fermentation in Red Wine
Fact Sheet WINEMAKING Malolactic fermentation in red wine Introduction Malolactic fermentation (MLF) is a secondary bacterial fermentation carried out in most red wines. Oenococcus oeni, a member of the lactic acid bacteria (LAB) family, is the main bacterium responsible for conducting MLF, due to its ability to survive the harsh conditions of wine (high alcohol, low pH and low nutrients) and its production of desirable wine sensory attributes. One of the important roles of MLF is to confer microbiological stability towards further metabolism of L-malic acid. Specifically, MLF removes the L-malic acid in wine that can be a carbon source for yeast and bacterial growth, potentially leading to spoilage, spritz and unwanted flavours. MLF can also be conducted in some wines to influence wine style. MLF often occurs naturally after the completion of primary fermentation or can also be induced by inoculation with a selected bacterial strain. Since natural or ‘wild’ MLF can be unpredictable in both time of onset and impact on wine quality, malolactic starter cultures are commonly used. In Australia, almost all red wines undergo MLF and 74% are inoculated with bacterial starter cultures (Nordestgaard 2019). This fact sheet provides practical information for induction of MLF in red wine. A separate fact sheet provides equivalent information for white and sparkling base wine. These should also be read in conjunction with another fact sheet, Achieving successful malolactic fermentation, which gives further practical guidelines for MLF induction, monitoring and management. Updated September 2020 Fact Sheet WINEMAKING Key parameters for a successful MLF in red wine Composition of red wine/must The main wine compositional factors that determine the success of MLF are alcohol, pH, temperature and sulfur dioxide (SO2) concentration. -
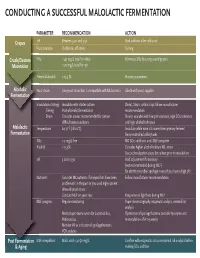
Conducting a Successful Malolactic Fermentation
CONDUCTING A SUCCESSFUL MALOLACTIC FERMENTATION PARAMETERPA RECOMMENDATION ACTION Grapes pH Between 3.20 and 3.50 Acid addition after cold soak FruitFru condition Visible rot, off odors Sorting Crush/Destem SO2SO < 40 mg/L total for white Minimize SO2 by using sound grapes Maceration < 70 mg/L total for red PotentialPPot alcohol < 13.5 % Harvest parameters Alcoholic YeastYeY a strain Use yeast strain that is compatible with ML bacteria Check with yeast supplier Fermentation InoculationIno strategy Inoculate with starter culture Direct, Step 1, or Build up: follow manufacturer Timing Post-alcoholic fermentation recommendation Strain Consider strains recommended for certain Strains available with low pH tolerance, high SO2 tolerance difficult wine conditions and high alcohol tolerance Malolactic TemperatureTem 64-71°F (18-22°C) Inoculate while wine still warm from primary ferment Fermentation Temp-controlled cellar/tanks SO2SO < 5 mg/L free NO SO2 additions until MLF complete AlcoholAlc < 13.5% Consider higher alcohol tolerant ML strain Use acclimatization steps for culture prior to inoculation pH 3.20 to 3.50 Acid adjustment if necessary (not recommended during MLF) Be alert to microbial spoilage issues if you have a high pH NNutrientsu Consider ML nutrients if vineyard lots have been Follow manufacturer recommendation problematic in the past or you used high nutrient demand yeast strain Conduct MLF on yeast lees Keep wine on light lees during MLF MMLFL progress Regular monitoring Paper chromatography, enzymatic analysis, external lab analysis Microscopic examination for Lactobacillus, If presence of spoilage bacteria consider lysozyme and Pediococcus re-inoculation after 2-3 weeks Monitor VA as indicator of spoilage bacteria PCR analysis Post Fermentation MLFML completion Malic acid < 30-50 mg/L Confirm with enzymatic assay or external lab analysis before & Aging making SO2 addition. -

Château Simone
Château Simone This historic estate, situated in the hills just south of Aix-en-Provence, has been in the hands of four generations of the Rougier family since 1830 and holds a virtual monopoly on the appellation of Palette. The property totals 120 hectares, with 28 under vine, of which 23 qualify for the Palette AOC. Its vineyards sit on limestone soils at elevations between 500 and 750 feet on the slopes of the Montaiguet, whose special microclimate is influenced by the encircling pine forests, the mass of the Mont Sainte-Victoire, and the Arc River. The vineyards were reconstituted after phylloxera and many vines are over a century old. In an industry that moves quicker than ever and presents ever-increasing novelty, an estate like Simone is an anchor of meaning and a lens into Provence's patrimony. Viticulture: • Farming: Ecocert Organic Certification Pending. Viticulture has always been practicing organic. • Treatments : No herbicides, chemical fertilizers, or synthetic treatments • Ploughing: Extensive ploughing and working of the soil by tractor. • Soils: Limestone Scree • Vines: Head-trained vines, many over 100-years old, that are replanted on a vine-by-vine basis to maintain a healthy vineyard • Yields: Old vines and extensive debudding lower yields and eliminate the need for a green harvest. • Harvest: Entirely manual harvest, grapes sorted in the vineyard and sorted again in the cellar • Purchasing: Always entirely estate fruit Vinification: Aging: • Fermentation: All wines are fermented spontaneously in large • Élevage: Palette wines are stored in foudres for 18 months; wooden foudres with temperature control for 2 weeks. red and white wines spend an additional year in neutral barrel. -

An Original Method for Producing Acetaldehyde and Diacetyl by Yeast
b r a z i l i a n j o u r n a l o f m i c r o b i o l o g y 4 7 (2 0 1 6) 949–954 ht tp://www.bjmicrobiol.com.br/ Industrial Microbiology An original method for producing acetaldehyde and diacetyl by yeast fermentation a a,∗ a b a,c Irina Rosca , Anca Roxana Petrovici , Mihai Brebu , Irina Stoica , Bogdan Minea , a Narcisa Marangoci a “Petru Poni” Institute of Macromolecular Chemistry, Advanced Research Center for Bionanoconjugates and Biopolymers, Aleea GrigoreGhica Voda, Iasi, Romania b SC Zeelandia SRL, R&D Department, Valea Lupului, Iasi, Romania c “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania a r a t i c l e i n f o b s t r a c t Article history: In this study a natural culture medium that mimics the synthetic yeast peptone glucose Received 2 June 2015 medium used for yeast fermentations was designed to screen and select yeasts capable Accepted 29 February 2016 of producing high levels of diacetyl and acetaldehyde. The presence of whey powder and Available online 25 July 2016 sodium citrate in the medium along with manganese and magnesium sulfate enhanced Associate Editor: Jorge Gonzalo both biomass and aroma development. A total of 52 yeasts strains were cultivated in two Farias Avendano different culture media, namely, yeast peptone glucose medium and yeast acetaldehyde- diacetyl medium. The initial screening of the strains was based on the qualitative reaction Keywords: of the acetaldehyde with Schiff’s reagent (violet color) and diacetyl with Brady’s reagent Acetaldehyde (yellow precipitate). -

An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes
International Journal of Environmental Research and Public Health Article An Unrecognized Hazard in E-Cigarette Vapor: Preliminary Quantification of Methylglyoxal Formation from Propylene Glycol in E-Cigarettes Parham Azimi 1, Zahra Keshavarz 1, Marianne Lahaie Luna 1,2, Jose Guillermo Cedeno Laurent 1 , Jose Vallarino 1, David C. Christiani 1 and Joseph G. Allen 1,* 1 Department of Environmental Health, Harvard T. H. Chan School of Public Health, Boston, MA 02115, USA; [email protected] (P.A.); [email protected] (Z.K.); [email protected] (M.L.L.); [email protected] (J.G.C.L.); [email protected] (J.V.); [email protected] (D.C.C.) 2 Occupational & Environmental Health Division, Dalla Lana School of Public Health, University of Toronto, Toronto, ON M5T 3M7, Canada * Correspondence: [email protected] Abstract: Up to 95% of the liquid volume in an e-cigarette consists of propylene glycol. Previous research has shown that propylene glycol can generate diacetyl and formaldehyde when heated. New research shows that propylene glycol can also generate methylglyoxal, an alpha di-carbonyl compound recently shown to cause epithelial necrosis at even lower concentrations than diacetyl, the flavoring chemical associated with bronchiolitis obliterans (“Popcorn Lung”). We analyzed chemical emissions from 13 JUUL pod flavors. Diacetyl and methylglyoxal was detected in 100% of samples 3 3 with median concentration (range) of 20 µg/m (less than limit of quantification: 54 µg/m ) and 4219 µg/m3 (677–15,342 µg/m3), respectively. We also detected acetaldehyde (median concentration: Citation: Azimi, P.; Keshavarz, Z.; 341 µg/m3) and propionaldehyde (median concentration: 87 µg/m3) in all samples. -

Influence of the Ph of Chardonnay Must on Malolactic Fermentation In
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by JKI Open Journal Systems (Julius Kühn-Institut) Vitis 45 (4), 197–198 (2006) Research Note plicate using a volume of 100 l for each trial. Commer- cial strains of Saccharomyces cerevisiae Lalvin Rhône Influence of the pH of Chardonnay 4600 and of Oenococcus oeni, Lalvin 31, were prepared according to the manufacture instructions. Bacteria were must on malolactic fermentation in- inoculated 16 h after the yeast inoculation when total and free SO were 28.0 and 6.4 mg·l-1, respectively. The fer- duced by bacteria co-inoculated with 2 mentations were monitored by analysis of ethanol produc- yeasts tion, L-malic acid consumption, yeast and bacteria cell concentrations. After AF, commercial MLF nutrient (Opti G. ZAPPAROLI1), E. TOSI2) and S. KRIEGER3) Malo Plus, Lallemand) was added according to the manu- facture instructions. Total acidity, sugars, ethanol, sulfite 1) Dipartimento Scientifico e Tecnologico, Università degli Studi and pH were determined using standard methods, organic di Verona, Verona, Italy acids were quantified using enzyme kits (Roche). Eight 2) Centro per la Sperimentazione in Vitivinicoltura, Provincia di biogenic amine (BA) (histamine, cadaverine, putrescine, Verona, Servizio Agricoltura, San Floriano, Verona, Italy phenylethylalanine, amylamine, isobutylamine, methyl- 3) Lallemand, office Korntal-Münchingen, Germany amine and isopropylamine) were determined as previously described (TORREA and ANCIN 2001). Data are average of K e y w o r d s : malolactic fermentation, yeast-bacteria two determinations. Cell counts were carried out plating co-inoculation, Chardonnay wine. on WL agar (Oxoid) medium for yeasts and MRS (Fluka) added 10 % tomato juice and 0.01 % actidione (Fluka) for Introduction: In wine, the success of the malolactic bacteria. -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Practical High-Acidity Winemaking Strategies for the Midwest
PRACTICAL HIGH-ACIDITY WINEMAKING STRATEGIES FOR THE MIDWEST DREW HORTON, ENOLOGY SPECIALIST UNIVERSITY OF MINNESOTA GRAPE BREEDING & ENOLOGY PROJECT GETTING STARTED •A BASIC UNDERSTANDING OF PH AND TOTAL ACIDITY IS INVALUABLE TO PRACTICAL WINE MAKING. PH VS. TA (TOTAL ACIDITY) • BOTH ARE A MEASURE OF THE RELATIVE STRENGTH OF ACIDITY IN WINE, BUT THEY DO NOT CORRELATE EXACTLY. • PH: THE INVERSE LOG OF HYDROGEN IONS IN A SOLUTION • PH: A LOGARITHMIC SCALE FROM 0 TO 14 • A PH OF 3.0 IS 10X HIGHER (OR “STRONGER”) THAN A PH OF 4.0. • TYPICALLY A PH BETWEEN 3.1 AND 3.6 IS DESIRABLE FOR WINE. PH VS. TA (TOTAL ACIDITY) • TOTAL ACIDITY IS AN EMPIRICAL MEASUREMENT OF ALL OF THE ACIDS IN A SOLUTION, OFTEN GIVEN IN GRAMS/LITER. • THE HIGHER THE ACIDITY, THE MORE TART A WINE WILL TASTE. • PH IS ABOUT MICROBIAL STABILITY, MOUTH FEEL, STRUCTURE, AND AGE-ABILITY OF A GIVEN WINE. • PH IS QUALITATIVE ACIDITY. TOTAL ACIDITY IS QUANTITATIVE… “… THE BEST THING FOR A VINEYARD ARE THE FOOTPRINTS OF THE WINEMAKER…” WINE IS MADE IN THE VINEYARD FIRST!!! • WINEMAKING STARTS IN THE VINEYARD. • VITICULTURAL PRACTICES AND HARVEST TIMING HAVE MAJOR EFFECTS • ACTIVE COLLABORATION BETWEEN VITICULTURIST AND WINEMAKER • VITICULTURE AND ENOLOGY ARE “TWO WINGS OF THE SAME BIRD”… • KNOWLEDGE OF ALL COLD-CLIMATE VARIETIES WILL HELP TO MAKE BALANCED WINES FROM THESE CHALLENGING VARIETIES. POST-VÉRAISON SUGAR/ACID CURVE ACIDS AND PH CHANGES •THROUGHOUT RIPENING, AS ACIDITY DECREASES, PH INCREASES… DETERMINING RIPENESS, AND WHEN TO PICK? RIPENESS IS MORE THAN A NUMBER. • AROMA AND FLAVOR • TEXTURE AND COLOR • CONDITION, OF VINEYARD, VINE, STEM, CLUSTER, BERRY, SKIN AND SEED… • OVERALL HEALTH, CROP LOAD, PEST ACTIVITY AND DISEASES • IMPENDING WEATHER AND PICKING CREW/MACHINERY AVAILABILITY USE THE LAB IN YOUR MOUTH AND NOSE. -

Malolactic Fermentation in Wine La Fermentation
MMALOLACTICALOLACTIC FFERMENTATIONERMENTATION IINN WWINEINE LLAA FFERMENTATIONERMENTATION MMALOLACTIQUEALOLACTIQUE DDUU VVININ LLAA FFERMENTACIÓNERMENTACIÓN MMALOLÁCTICAALOLÁCTICA DDELEL VVINOINO LLAA FFERMENTAZIONEERMENTAZIONE MMALOLATTICAALOLATTICA DELDEL VVINOINO BBIOLOGISCHERIOLOGISCHER SSÄUREABBAUÄUREABBAU ININ WEINWEIN AAPPEL-MELKSUURGISTINGPPEL-MELKSUURGISTING IINN WWYNYN A FFERMENTAÇÃOERMENTAÇÃO MMALOLÁCTICAALOLÁCTICA DDOO VINHOVINHO MMALOLAKTIALOLAKTICCNAˇ NA FFERMENTACIJAERMENTACIJA VINAVINA MALOLACTIC FERMENTATION IN WINE UNDERSTANDING THE SCIENCE AND THE PRACTICE Magali Bou (France) Piet Loubser (Republic of South Africa) Dr. Neil Brown (U.S.A.) Dr. Rich Morenzoni (U.S.A.) Dr. Peter Costello (Australia) Dr. Antonio Palacios (Spain) Dr. Richard Degré (Canada) Dr. Chris Powell (United Kingdom) Wilfried Dieterich (Germany) Katie Scully Specht (U.S.A.) Sigrid Gertsen-Briand (Canada) Gordon Specht (U.S.A.) Samantha Kollar (U.S.A.) Didier Theodore (France) Dr. Sibylle Krieger (Germany) Dr. Sylvie Van Zandycke (Belgium) Annamarie Kyne (U.S.A.) Scientifi c Editor: Dr. Rich Morenzoni Managing Editor: Katie Scully Specht Published by OCTOBER 2005 ii MALOLACTIC FERMENTATION IN WINE Production Coordinator: Claude Racine Copy editing: Judith Brown Designer: François Messier Printing: Les Impressions Au Point Certain research published or cited in this publication was funded in whole or in part by Lallemand Inc. DISCLAIMER Lallemand has compiled the information contained herein and, to the best of its knowledge, the information is true and -

Mastering Malolactic Fermentation – How to Manage the Nutrition of Wine
Mastering malolactic MAGALI fermentation – how to DÉLÉRIS-BOU & SIBYLLE manage the nutrition of KRIEGER-WEBER Lallemand SAS, Blagnac, wine bacteria and minimise France the effect of inhibitors Keywords: Malolactic fermentation, nutrition, inhibitors. 1. Introduction Malic acid penetrates into the cell in its anionic form to be Wine bacteria are characterised as having complex nutritional needs. decarboxylated into lactic acid in the cell’s cytoplasm. The de In this review, we will detail the wine bacteria’s requirements for carboxylation allows the consumption of an intracellular proton and carbon, and for nutrients containing nitrogen, vitamins and minerals. the expulsion of protons by lactate/H+ symporters. A proton gradient When a must or wine lacks certain nutritional elements, it can have – or “proton-motive force” – from the wine medium towards the cell a major impact on malolactic fermentation (MLF). Therefore, it is interior maintains the intracellular pH of the bacteria (about 6.0) and important to understand the nutritional needs of wine bacteria and to leads to forming energy as ATP. learn about the tools that are available to the winemaker for successful MLF. 2.2.2 Citric acid 2. Wine bacteria have complex nutritional Citric acid, an important component of grape must and wine, has a concentration that varies from 0.1 to 0.7 g/L. The degradation requirements pathway of citric acid by lactic bacteria leads to the formation of 2.1 Carbohydrate metabolism three types of compounds: acetic acid, lipids and acetoinic derivatives (acetoin, butanediol and diacetyl). The metabolism of citric acid is In wine, sugars are the primary source of energy for lactic bacteria, also a source of energy for O. -

Recommendation from the Scientific Committee on Occupational Exposure Limits for Diacetyl
Employment, Social Affairs & Inclusion SCOEL Recommendation on Diacetyl Recommendation from the Scientific Committee on Occupational Exposure Limits for Diacetyl SCOEL/SUM/149 June 2014 June 2014 1 Employment, Social Affairs & Inclusion SCOEL Recommendation on Diacetyl Table of Contents 1. Substance identification, physico-chemical properties.............................................. 3 2. Occurrence/use and occupational exposure ........................................................... 3 2.1. Occurrence and use ...................................................................................... 3 2.2. Occupational exposure .................................................................................. 4 2.3. Methods of exposure monitoring and analysis .................................................. 5 3. Health significance ............................................................................................. 6 3.1. Toxicokinetics .............................................................................................. 7 3.1.1. Human data ........................................................................................... 7 3.1.2. Animal data ........................................................................................... 7 3.1.3. Biological monitoring ............................................................................... 8 3.2. Acute toxicity ............................................................................................... 8 3.2.1. Human data ..........................................................................................