Guide to White Wine Making
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brewing Industry - Preparation of Isinglass Finings
Solutions for Your TOUGHEST MIXING Applications in FOOD Brewing Industry - Preparation of Isinglass Finings THE FIRST NAME IN HIGH SHEAR MIXERS Brewing Industry - Preparation of Isinglass Finings Lager beer is brewed using “bottom fermenting” yeast which sinks during fermentation. A storage period follows to ensure all the yeast has sunk to the bottom. Demands for a shorter storage period and increased productivity in large-scale breweries has led to the use of finings to accelerate sedimentation. The use of isinglass finings was originally confined to traditional British and Irish beers, brewed with “top fermenting” yeast which rises during fermentation. Spent yeast and other solids are precipitated by the addition of finings, either in the barrel (with traditional “cask conditioned” beer) or in bulk prior to filtering. Isinglass is a protein obtained from several varieties of fish. When added to the beer, the isinglass particles attract the yeast and tannins. The finings sink to the bottom, precipitating the solids to leave the beer clear and bright. The Process Isinglass can be supplied in a number of forms: • Ready-to-use liquid. • Concentrated liquid. • Dried floculated particles. • Powder. • Freeze-dried powder/granules. Although liquid forms are easy to use, there are advantages in buying finings in dried form: • Improved shelf life and temperature tolerance. • Solutions can be prepared to meet demand. • Reduced storage requirements. • Formulation can be adjusted to give optimum results in a particular type of beer. Solutions are generally prepared separately before addition to the beer. A typical process using a conventional agitator would be as follows: • The vessel is charged with liquor and the agitator is started. -

Concepts in Wine Chemistry
THIRD EDITION Concepts IN Wine Chemistry YAIR MARGALIT, Ph.D. The Wine Appreciation Guild San Francisco Contents Introduction ix I. Must and Wine Composition 1 A. General Background 3 B. Sugars 5 C. Acids 11 D. Alcohols 22 E. Aldehydes and Ketones 30 F. Esters 32 G. Nitrogen Compounds 34 H. Phenols 43 I. Inorganic Constituents 52 References 55 n. Fermentation 61 A. General View 63 B. Chemistry of Fermentation 64 C. Factors Affecting Fermentation 68 D. Stuck Fermentation 77 E. Heat of Fermentation 84 F. Malolactic Fermentation 89 G. Carbonic Maceration 98 References 99 v III. Phenolic Compounds 105 A. Wine Phenolic Background 107 B. Tannins 120 C. Red Wine Color 123 D. Extraction of Phenolic Compounds from Grapes 139 References 143 IV. Aroma and Flavor 149 A. Taste 151 B. Floral Aroma 179 C. Vegetative Aroma 189 D. Fruity Aroma 194 E. Bitterness and Astringency 195 F. Specific Flavors 201 References 214 V. Oxidation and Wine Aging 223 A. General Aspects of Wine Oxidation 225 B. Phenolic Oxidation 227 C. Browning of White Wines 232 D. Wine Aging 238 References 253 VI. Oak Products 257 A. Cooperage 259 B. Barrel Aging 274 C. Cork 291 References 305 vi VH. Sulfur Dioxide 313 A. Sulfur-Dioxide as Food Products Preservative 315 B. Sulfur-Dioxide Uses in Wine 326 References 337 Vm. Cellar Processes 341 A. Fining 343 B. Stabilization 352 C. Acidity Adjustment 364 D. Wine Preservatives 372 References 382 IX. Wine Faults 387 A. Chemical Faults 389 B. Microbiological Faults 395 C. Summary ofFaults 402 References 409 X. -

Basic Definitions and Tips for Winemaking
Presque Isle Wine Cellars “Serving the Winemaker Since 1964” (814) 725-1314 www.piwine.com Basic Winemaking Terms & Tips Definitions & Tips: Not all-inclusive but hopefully helpful. Email us your favorites; maybe we’ll include them in the next edition. Acid Reduction - Reducing the acid in juice or wine to an acceptable level. It is usually measured as tartaric acid and requires a testing apparatus and reagents. Good levels are typically in a range of 0.6 to 0.8 percent acid, depending on the wine. More technically the reading is read as grams per liter. Therefore 0.6 percent would be 6.0 g/l. Acidulation or Acidification - Raising the acid level of juice, wine or sometimes water by adding some type of acid increasing additive or blending with a higher acid juice or wine. Acidified or Acidulated Water - Water to which acid (most commonly citric acid) has been added. It is a way to reduce sugar in a juice that is too high in sugar without diluting (thus reducing) the acid level of that juice. Additives - Things added to wine to enhance quality or possibly fix some type of flaw. There are many additives for many situations and it is wise to gain at least some basic knowledge in this area. Alcohol - Obviously one of the significant components of wine. Yeast turns sugar to alcohol. Rule of thumb says for each percentage of sugar in a non-fermented juice, the alcohol will be half. For example 21% sugar should ferment out to an alcohol level of about 11.5 to 12%. -

Domaine Luneau-Papin Muscadet from Domaine Luneau-Papin
Domaine Luneau-Papin Muscadet from Domaine Luneau-Papin. Pierre-Marie Luneau and Marie Chartier. Photo by Christophe Bornet. Pierre and Monique Luneau. Photo by Christophe Bornet. Profile Pierre-Marie Luneau heads this 50-hectare estate in Le Landreau, a village in the heart of Muscadet country, where small hamlets dot a landscape of vineyards on low hills. Their estate, also known as Domaine Pierre de la Grange, has been in existence since the early 18th century when it was already planted with Melon de Bourgogne, the Muscadet appellation's single varietal. After taking over from his father Pierre in 2011, Pierre-Marie became the ninth generation to make wine in the area. Muscadet is an area where, unfortunately, a lot of undistinguished bulk wine is produced. Because of the size of their estate, and of the privileged terroir of the villages of Le Landreau, Vallet and La Chapelle Heulin, the Luneau family has opted for producing smaller cuvées from their several plots, which are always vinified separately so as to reflect their terroir's particular character. The soil is mainly micaschist and gneiss, but some plots are a mix of silica, volcanic rocks and schist. The estate has a high proportion of old vines, 40 years old on average, up to 65 years of age. The harvest is done by hand -also a rarity in the region- to avoid any oxidation before pressing. There is an immediate light débourbage (separation of juice from gross lees), then a 4-week fermentation at 68 degrees, followed by 6 months of aging in stainless-steel vats on fine lees. -

Structure in Wine Steiia Thiast
Structure in Wine steiia thiAst What is Structure? • So what is this thing, structure? It*s the sense you have that the wine has a well-established form,I think ofit as the architecture ofthe wine. A wine with a great structure will often remind me ofthe outlines of a cathedral, or the veins in a leaf...it supports, and balances the fiuit characteristics ofthe wine. The French often describe structure as the skeleton ofthe wine, as opposed to its flavor which they describe as the flesh. • Where does structure come firom? In white wines, it usually comes from alcohol or acidity; in red wines, it comes from a combination of acidity and tannin, a component in the grapes' skins and seeds. Thus, wines with a lot of tannin (like cabernet) also have a lot of structure. Beaujolais is made from gamay which does not have much tannin. As a result, Beaujolais can lack structure; it feels soft, flat or simple in the mouth (though its flavors can certainly still be attractive). • While structure is hard to articulate, you can easily taste or sense it —^and the lack of it. • Understanding structure is critical to understanding any ofthe ''powerful" red varieties: cabernet sauvignon, merlot, syrah, nebbiolo, tempranillo, and malbec, to name a few. I just don't think you can understand these wines unless you understand structure, and how it frames and focuses the powerful rush of fruit. It adds freshness, and a "lightness" to the density ofripe fiuit. Structure matters when pairing wine and food. Foods with a lot of structure themselves— like a meaty, thick steak-need wines with commensurate structure (like cabernet), or the food experience can dwarfthe wine experience. -

Malolactic Fermentation in Red Wine
Fact Sheet WINEMAKING Malolactic fermentation in red wine Introduction Malolactic fermentation (MLF) is a secondary bacterial fermentation carried out in most red wines. Oenococcus oeni, a member of the lactic acid bacteria (LAB) family, is the main bacterium responsible for conducting MLF, due to its ability to survive the harsh conditions of wine (high alcohol, low pH and low nutrients) and its production of desirable wine sensory attributes. One of the important roles of MLF is to confer microbiological stability towards further metabolism of L-malic acid. Specifically, MLF removes the L-malic acid in wine that can be a carbon source for yeast and bacterial growth, potentially leading to spoilage, spritz and unwanted flavours. MLF can also be conducted in some wines to influence wine style. MLF often occurs naturally after the completion of primary fermentation or can also be induced by inoculation with a selected bacterial strain. Since natural or ‘wild’ MLF can be unpredictable in both time of onset and impact on wine quality, malolactic starter cultures are commonly used. In Australia, almost all red wines undergo MLF and 74% are inoculated with bacterial starter cultures (Nordestgaard 2019). This fact sheet provides practical information for induction of MLF in red wine. A separate fact sheet provides equivalent information for white and sparkling base wine. These should also be read in conjunction with another fact sheet, Achieving successful malolactic fermentation, which gives further practical guidelines for MLF induction, monitoring and management. Updated September 2020 Fact Sheet WINEMAKING Key parameters for a successful MLF in red wine Composition of red wine/must The main wine compositional factors that determine the success of MLF are alcohol, pH, temperature and sulfur dioxide (SO2) concentration. -

Chardonnay Lees Management with Extralyse (ARC) Blenheim Vineyards Submitted by Kirsty Harmon Summary This Study Examined the Im
Chardonnay Lees Management with Extralyse (ARC) Blenheim Vineyards Submitted by Kirsty Harmon Summary This study examined the impact of lees stirring and batonnage enzyme addition during Chardonnay aging on the chemical and sensory qualities of the wine. It is a companion study to Blenheim’s Chardonnay Lees Management (2016), which compared the effects of not stirring Chardonnay to stirring Chardonnay. Chardonnay juice was fermented in barrels, and afterwards two different treatments were imposed: stirred, and stirred with Extralyse (Laffort). Stirring occurred once per week for 8 weeks. No major chemical differences could be observed between the finished wines. Wine tended to become more cold stable over time. Additionally, increased bentonite additions to become heat stable were necessary after aging. In general, people often could not distinguish between stirring and stirring with Extralyse. When people could distinguish, there appeared to be a slight preference for wine made with Extralyse. The descriptors used generally did not help elucidate which qualities in wine were affected by stirring. There may be a small tendency for Extralyse to enhance Fruit Intensity and Depth of Flavor, but these tendencies were weak. However, the stirring regime for this study was relatively short (8 weeks). In the future, more realistic stirring regimes should be implemented to see whether differences tend to increase over time. Introduction Marchal et al. (2011) provide an excellent brief review of yeast autolysis in their introduction. Lees are mainly composed of yeast, bacteria, tartaric acid, polysaccharides, and protein-tannin complexes (Zoecklein 2013). Heavy lees generally refers to lees which precipitate 24 hours after fermentation (generally grape particles and large complexes of other lees particulates), and can often lead to off- aromas in wine. -
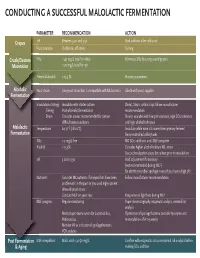
Conducting a Successful Malolactic Fermentation
CONDUCTING A SUCCESSFUL MALOLACTIC FERMENTATION PARAMETERPA RECOMMENDATION ACTION Grapes pH Between 3.20 and 3.50 Acid addition after cold soak FruitFru condition Visible rot, off odors Sorting Crush/Destem SO2SO < 40 mg/L total for white Minimize SO2 by using sound grapes Maceration < 70 mg/L total for red PotentialPPot alcohol < 13.5 % Harvest parameters Alcoholic YeastYeY a strain Use yeast strain that is compatible with ML bacteria Check with yeast supplier Fermentation InoculationIno strategy Inoculate with starter culture Direct, Step 1, or Build up: follow manufacturer Timing Post-alcoholic fermentation recommendation Strain Consider strains recommended for certain Strains available with low pH tolerance, high SO2 tolerance difficult wine conditions and high alcohol tolerance Malolactic TemperatureTem 64-71°F (18-22°C) Inoculate while wine still warm from primary ferment Fermentation Temp-controlled cellar/tanks SO2SO < 5 mg/L free NO SO2 additions until MLF complete AlcoholAlc < 13.5% Consider higher alcohol tolerant ML strain Use acclimatization steps for culture prior to inoculation pH 3.20 to 3.50 Acid adjustment if necessary (not recommended during MLF) Be alert to microbial spoilage issues if you have a high pH NNutrientsu Consider ML nutrients if vineyard lots have been Follow manufacturer recommendation problematic in the past or you used high nutrient demand yeast strain Conduct MLF on yeast lees Keep wine on light lees during MLF MMLFL progress Regular monitoring Paper chromatography, enzymatic analysis, external lab analysis Microscopic examination for Lactobacillus, If presence of spoilage bacteria consider lysozyme and Pediococcus re-inoculation after 2-3 weeks Monitor VA as indicator of spoilage bacteria PCR analysis Post Fermentation MLFML completion Malic acid < 30-50 mg/L Confirm with enzymatic assay or external lab analysis before & Aging making SO2 addition. -

The Path to Bottling
The Path to Bottling www.laffort.com YOUR ACCESS TO 120 YEARS OF WINEMAKING INNOVATION Save it in your favourites now for 24/7 access to ! PRODUCT INFORMATION SHEETS QUALITY DOCUMENTS ( HACCP & ISO ) PRODUCT SAFETY SHEETS RESEARCH PAPERS DECISION MAKING TOOLS CATALOGUE DOWNLOADS PERSONALISED NUTRITION PUBLISHED ARTICLES CALCULATOR CERTIFICATES OF ANALYSIS ORGANIC CERTIFICATIONS TRAINING VIDEOS LAFFORT® PLANT BASED INNOVATIONS LAFFORT® unrivalled technical resources is delivering the most scientifically advanced oenological solutions from plant derived products. VEGAN FRIENDLY & ORGANIC THEY’RE NOT JUST OPTIONS THEY’RE SUPERIOR SOLUTIONS These symbols are a guide to your LAFFORT® products properties. GANI TAL OR LERGE OR C E IG L N G I A E N V A L E L E S E R UIT ABLE RGEN F F R E E Organic certification bodies have different criteria for certification and products may differ from one certification body to another. Please contact your certifying agent to confirm a products organic certification. PROTECTING YOUR WHITE WINE MANAGEMENT WHITE WINE PROTECTING YOUR WHITE WINE - A TRADITIONAL APPROACH White wines are vulnerable to oxidation and microbial changes post alcoholic fermentation. Microbial and anti-oxidative control of white wines is a first step to getting wines ready for bottling and/or storage. Threats of oxygen on finished white wines; • Proliferation of acetic acid bacteria. • Proliferation of Brettanomyces bruxellensis. • Browning caused by the oxidation of hydroxycinnamic acids and key phenolic acids. • Oxidation of aroma producing thiols rendering them non volatile. To prevent the oxidation of these phenolic compounds, an anti-oxidative mechanism needs to be put in place. -

Sugar in Wine Join Us Every Friday Evening from 5Pm to 8Pm
July 2014 A WINE ENTHUSIAST’S MONTHLY JOURNEY THROUGH MONTEREY’S WINE COUNTRY COMING EVENTS Every Fridays Wine Bar A Taste of Monterey 5:00pm-8:00pm* Sugar in Wine Join us every Friday evening from 5pm to 8pm. Start your weekend off More than a Sweetener with a relaxing view, live music, great food and a Sugars occur naturally in fruits, grapes included. So, it should come as no surprise that there’s glass of Monterey’s finest. sugar in that glass of wine you’re sipping from. And, no, we’re just talking about the overly sweet taste in a ruby port-style wine. We’re talking about the presence of sugars in all wines. In fact, sugars are required in order for the fermentation process in winemaking to occur, so really they are an important part of the process. But, what about this thing you’ve heard of called the “re- sidual sugar” content of a particular wine? Does it really mean leftover sugar, as the name would imply? Yes…in a way, but we’ll come back to this topic. Store Hours Wine grapes accumulate sugars while they are on the vine during the photosynthesis process. A Taste of Monterey Cannery Row At harvest time, a grape will be composed of 15 to 25% different sugar compounds. Then, later Sun-Wed 11am-7pm during the fermentation process, the introduction of yeast breaks down and converts most of Thu-Sat 11am-8pm the sugars into alcohol and carbon dioxide. Once an alcohol level *No new member tastings is reached during fermentation, the remaining sugars will effec- after 6:00pm tively kill off the yeast. -

Enartis News – FAQ on Wine Fining
ENARTIS NEWS FAQ ON WINE FINING WHY FINING? HOW TO CHOOSE THE RIGHT FINING Fining agents can be used for many purposes in AGENT winemaking, including clarification, filterability Bench trials are essential to determine what fining improvement, prevention of haze and sediment agents to use and their dosages. To set up bench formation, organoleptic profile improvement, color trials, follow these steps: adjustment and removal of undesirable elements or flavors. The fining process is therefore a crucial • Prepare fining agent solution in water as stage in the production of all wine types. recommended on the technical data sheet. For liquid products, use solution as is or dilute, if HOW DOES FINING WORK? necessary. Each fining agent has specific properties and reacts • Label each sample bottle. Keep one untreated with various wine constituents depending on its sample as a control. origin, density of charge, molecular weight and • Fill samples with wine and leave some space for chemical properties. the addition. Fining involves two crucial reactions: • Add the fining agent solution. Flocculation: molecular interactions based on • Mix immediately after addition. Top each bottle charge, chemical bonds, absorption or adsorption with wine and mix again. of compounds and formation of flocculates. • Store wine at winery temperature for settling Sedimentation: flocculates formed are not soluble (usually 1-2 days). and heavier than wine/juice. They settle with time. • Evaluate the results (turbidity, volume of lees, WHAT ARE THE MAIN FACTORS THAT color, sensory, stability, etc.) INFLUENCE FINING EFFECTIVENESS? HOW TO EVALUATE FINING RESULTS Product preparation and addition, temperature, pH, Depending on the goal, several parameters can be wine redox potential and previous fining treatments used to evaluate the results of a fining treatment: are factors that can influence the effectiveness of turbidity, volume of lees, filterability, color intensity fining. -

Phenolic Compounds As Markers of Wine Quality and Authenticity
foods Review Phenolic Compounds as Markers of Wine Quality and Authenticity Vakare˙ Merkyte˙ 1,2 , Edoardo Longo 1,2,* , Giulia Windisch 1,2 and Emanuele Boselli 1,2 1 Faculty of Science and Technology, Free University of Bozen-Bolzano, Piazza Università 5, 39100 Bozen-Bolzano, Italy; [email protected] (V.M.); [email protected] (G.W.); [email protected] (E.B.) 2 Oenolab, NOI Techpark South Tyrol, Via A. Volta 13B, 39100 Bozen-Bolzano, Italy * Correspondence: [email protected]; Tel.: +39-0471-017691 Received: 29 October 2020; Accepted: 28 November 2020; Published: 1 December 2020 Abstract: Targeted and untargeted determinations are being currently applied to different classes of natural phenolics to develop an integrated approach aimed at ensuring compliance to regulatory prescriptions related to specific quality parameters of wine production. The regulations are particularly severe for wine and include various aspects of the viticulture practices and winemaking techniques. Nevertheless, the use of phenolic profiles for quality control is still fragmented and incomplete, even if they are a promising tool for quality evaluation. Only a few methods have been already validated and widely applied, and an integrated approach is in fact still missing because of the complex dependence of the chemical profile of wine on many viticultural and enological factors, which have not been clarified yet. For example, there is a lack of studies about the phenolic composition in relation to the wine authenticity of white and especially rosé wines. This review is a bibliographic account on the approaches based on phenolic species that have been developed for the evaluation of wine quality and frauds, from the grape varieties (of V.