Joseph Onoufriou Phd Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Document in PDF Format
Report No. 37 (2008–2009) to the Storting Report No. Report No. 37 (2008–2009) to the Storting Published by: Norwegian Ministry of the Environment Integrated Management of the Marine Environment of the Norwegian Sea Internet address: www.government.no Front cover illustration: Watercolour by Ørnulf Opdahl, photographed by Silje Gripsrud Printed by: 07 Gruppen AS – 09/2009 – Impression 1000 Integrated Management of the Marine Environment of the Norwegian Sea ER JØM KE IL T M 2 4 9 1 7 3 Trykksak Ørnulf Opdahl: The Norwegian Sea (watercolour) Ørnulf Opdahl (born in 1944 in Ålesund) is one of Norway’s most distinguished artists. His work is inspired by the ever-changing landscape along the Norwegian coast, and his dramatic depictions of coastal landscapes have caused him to be described as a contemporary Romantic painter. The Norwegian Sea was painted during a cruise with the research vessel G.O. Sars in 2004. Ørnulf Opdahl accompanied a team of 60 researchers from 13 countries on a two-month expedition to the Mid-Atlantic Ridge as part of the MAR-ECO project. The purpose of the expedition was to enhance understanding of the distribution and ecology of marine animal communities. In previous centuries, before the invention of photography, artists often accompanied scientifi c expeditions to document their scientifi c fi ndings. Ørnulf Opdahl’s watercolour was photographed by Silje Gripsrud Report No. 37 (2008–2009) to the Storting Integrated Management of the Marine Environment of the Norwegian Sea Translation from the Norwegian. For information only. Table of contents 1 Summary . 7 3.3.4 The edge of the continental shelf . -

Marine Current Energy Conversion
Marine Current Energy Conversion Resource and Technology MÅRTEN GRABBE UURIE 309-09L ISSN 0349-8352 Division of Electricity Department of Engineering Sciences Uppsala, December 2008 Abstract Research in the area of energy conversion from marine currents has been car- ried out at the Division of Electricity for several years. The focus has been to develop a simple and robust system for converting the kinetic energy in freely flowing water to electricity. The concept is based on a vertical axis turbine di- rectly coupled to a permanent magnet synchronous generator that is designed to match the characteristics of the resource. During this thesis work a pro- totype of such a variable speed generator, rated at 5 kW at 10 rpm, has been constructed to validate previous finite element simulations. Experiments show that the generator is well balanced and that there is reasonable agreement be- tween measurements and corresponding simulations, both at the nominal op- erating point and at variable speed and variable load operation from 2–16 rpm. It is shown that the generator can accommodate operation at fixed tip speed ratio with different fixed pitch vertical axis turbines in current velocities of 0.5–2.5 m/s. The generator has also been tested under diode rectifier opera- tion where it has been interconnected with a second generator on a common DC-bus similar to how several units could be connected in offshore operation. The conditions for marine current energy conversion in Norway have been investigated based on available data in pilot books and published literature. During this review work more than 100 sites have been identified as interest- ing with an estimated total theoretical resource—i.e. -

Connectivity Among Subpopulations of Norwegian Coastal Cod Impacts of Physical-Biological Factors During Egg Stages
Connectivity among subpopulations of Norwegian Coastal cod Impacts of physical-biological factors during egg stages Mari Skuggedal Myksvoll Dissertation for the degree of Philosophiae Doctor (PhD) Geophysical Institute, University of Bergen, Norway January 2012 Connectivity among subpopulations of Norwegian Coastal cod Impacts of physical-biological factors during egg stages Mari Skuggedal Myksvoll Institute of Bjerknes Center for Marine Research Climate Research Dissertation for the degree of Philosophiae Doctor (PhD) Geophysical Institute, University of Bergen, Norway January 2012 Outline This thesis consists of an introduction and four papers. The introduction provides a scientic background of the population structure of Atlantic cod stocks in Norwegian Waters and the research history of fjord dynamics (Section 1). Section 2 states the motivation for the study and the most important results from the papers. A discussion follows focusing on the implications of the present results (Section 3) and perspectives for future research are stated in Section 4. • Paper I: Retention of coastal cod eggs in a fjord caused by interactions between egg buoyancy and circulation pattern Myksvoll, M.S., Sundby, S., Ådlandsvik, B. and Vikebø, F. (2011) Marine and Coastal Fisheries, 3, 279-294. • Paper II: Importance of high resolution wind forcing on eddy activity and particle dispersion in a Norwegian fjord Myksvoll, M.S., Sandvik, A.D., Skarðhamar, J. and Sundby, S. (2012) Submitted to Estuarine, Coastal and Shelf Sciences • Paper III: Eects of river regulations on fjord dynamics and retention of coastal cod eggs Myksvoll, M.S., Sandvik, A.D., Asplin, L. and Sundby, S. (2012) Manuscript • Paper IV: Modeling dispersal of eggs and quantifying connectivity among Norwegian Coastal cod subpopulations Myksvoll, M.S., Jung, K.-M., Albretsen, J. -

A Vibrant City on the Edge of Nature 2019/2020 Welcome to Bodø & Salten
BODØ & SALTEN A vibrant city on the edge of nature 2019/2020 Welcome to Bodø & Salten. Only 90 minutes by plane from Oslo, between the archipelago and south, to the Realm of Knut Hamsun in the north. From Norway’s the peaks of Børvasstidene, the coastal town of Bodø is a natural second biggest glacier in Meløy, your journey will take you north communications hub and the perfect base from which to explore via unique overnight accommodations, innumerable hiking trails the region. The town also has gourmet restaurants, a water and mountain peaks, caves, fantastic fishing spots, museums park, cafés and shopping centres, as well as numerous festivals, and cultural events, and urban life in one of the country’s including the Nordland Music Festival, with concerts in a beautiful quickest growing cities, Bodø. In the Realm of Hamsun in the outdoor setting. Hamsun Centre, you can learn all about Nobel Prize winner Knut Hamsun`s life and works, and on Tranøy, you can enjoy open-air This is a region where you can get close to natural phenomena. art installations against the panoramic backdrop of the Lofoten There is considerable contrast – from the sea to tall mountains, mountain range. from the midnight sun to the northern lights, from white sandy beaches to naked rock. The area is a scenic eldorado with wild On the following pages, we will present Bodø and surroundings countryside and a range of famous national parks. Here, you can as a destination like no other, and we are more than happy immerse yourself in magnificent natural surroundings without to discuss any questions, such as possible guest events and having to stand in line. -

Variable Predator–Prey Relations in Zooplankton Overwintering in Subarctic Fjords Stig Skreslet , Marina Espinasse , Ketil Olsen & Boris D
RESEARCH ARTICLE Variable predator–prey relations in zooplankton overwintering in Subarctic fjords Stig Skreslet , Marina Espinasse , Ketil Olsen & Boris D. Espinasse Faculty for Biosciences and Aquaculture, Nord University, Bodø, Norway Abstract Keywords Zooplankton predator–prey relations in northern Norwegian fjords are highly Crustacea; Polychaeta; Chaetognatha; variable in time and space, and the mechanisms driving this variability are still Coelenterata; advection; predation poorly understood. Replicate Juday net sampling in October and February from 1983 to 2005, which included five repeated tows from bottom to surface, was Correspondence conducted in Saltfjord and Mistfjord, northern Norway. The time-series pro- Stig Skreslet, Faculty for Biosciences and vided evidence of in situ variability in species abundance, as well as seasonal Aquaculture, Nord University, PO Box 614, NO-8622 Mo i Rana, Norway. E-mail: stig. and interannual changes in standing stock abundance. The shallow sill of one [email protected] fjord caused accumulation of coastal water in the fjord’s basin, while the other fjord’s deeper sill selected denser water of Atlantic origin from the same open Abbreviations shelf habitat. The selective advection caused differences in the immigration CIV, CV, CVI: copepodid stages of species recruiting to the fjords’ specific overwintering communities of zoo- DI: deviation index plankton. Statistical analyses of the cumulated replicate data indicated signifi- DVM: diurnal vertical migration cant in situ variability in the spatial density of species. Cases with an abundance NAC: Norwegian Atlantic Current NCC: Norwegian Coastal Current of carnivores relating positively to other species probably resulted from the carnivores’ attraction to patches with concentrations of prey. Interspecific neg- ative density relations likely indicated either predator avoidance or substantial trophic activity during the sampling. -

Saltstraumen Ved Bodø
13 Saltstraumen ved Bodø 1 Den mest kjente tidevannsstrømmen i Norge er uten tvil Saltstraum- en som ligger tre mils vei sørøst for Bodø. Den er beskrevet i Nordlands Trompet av Petter Dass, i de klassiske reiseskildringene fra Nordland av Schøyen (1915) og Ytteren (1944) og i mange andre skrifter og bøker. Gjennom fire smale sund blir den store Skjerstadfjorden fyllt opp til flo og deretter tømt ned til fjære hver eneste tidevannssyklus. Hovedløpet for strømmen går mellom Straumøya og Knaplundøya. Det deles i to av Storholmen (fig. 13.3). Øst for Storholmen, hvor større skip kan passere, er åpningen omkring 125 meter bred og 30 meter dyp på det smaleste. Løpet mellom Storholmen og Straumøya er omkring 60 meter bredt og 25 meter dypt. Gjennom disse to løpene renner den mektige Saltstraumen. Litt lengere nord finner en Godøystraumen i det grunne og smale sund- et mellom Knaplundøya og fastlandet. Dette sundet går nesten tørt ved lavvann. Gjennom et annet trangt sund mellom Straumøya og fastland- et lenger sør, renner Sundstraumen eller Sundanstraumen som den også kalles. Dette sundet kan bare passeres med små båter. Vanntransporten gjennom de to sidestrømmene Godøystraumen og Sundstraumen, er liten i forhold til selve Saltstraumen. Flateareal av Skjerstadfjorden med tilstøtende fjordarmer er omkring 235 kvadratkilometer og forskjellen på flo og fjære er opptil 2,0 meter in- nerst i fjorden. Det betyr at ca. 470 milloner kubikkmeter sjø skal passere gjennom innløpene i hver tidevannssyklus. Når strømmen er på det ster- keste, renner det omkring 33 tusen kubikkmeter gjennom innløpene per sekund. Det er hele 10 ganger større vanntransport enn i Glomma under stor vårflom, men likevel mye mindre enn i Moskstraumen utenfor Lofot- odden (se kapittel 12). -
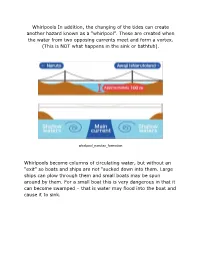
Whirlpools in Addition, the Changing of the Tides Can Create Another Hazard Known As a “Whirlpool”
Whirlpools In addition, the changing of the tides can create another hazard known as a “whirlpool”. These are created when the water from two opposing currents meet and form a vortex. (This is NOT what happens in the sink or bathtub). whirlpool_narutao_formation Whirlpools become columns of circulating water, but without an “exit” so boats and ships are not “sucked down into them. Large ships can plow through them and small boats may be spun around by them. For a small boat this is very dangerous in that it can become swamped – that is water may flood into the boat and cause it to sink. Whether or not the whirlpool appears and how rapidly it spins are a function of the tides. High tides and especially spring tides produce the most intense whirlpools. The figures given below are “highs”. In some cases, these have become tourist attractions with boats taking passengers out to see the whirlpool “up close” whirlpool_narutao_tourists The largest whirlpools are Saltstraumen (23 mph) in Norway, Whirlpool_Saltstraumen_Norway Moskstraumen (17.3) also in Norway, although there are those who think this is more an “eddy” than a whirl pool and Corryvreckan in Scotland found off the west coast between Jura and Scarpa. It has speeds of about 12 mph Whirlpool_Corryvreckan A dramatic encounter with Corryvreckan can be seen in the film I Know Where I’m Going. On the US/Canadian border lies Old Sow (17.1 mph) between Deer Island in New Brunswick and Moose Island, Eastport Maine. The US Coast Guard Station in Eastport regularly rescues boats that have gotten too close and do not have enough power to move them out of the current. -
Saltstraumentext by Christian Skauge Photos by Stein Johnsen Translated and Edited by Arnold Weisz
Macro life abounds. LEFT: Most of the rockfaces are well covered with a variety of marine life. These soft corals are quite common in nordic waters. Wet’n wild at the Arctic circle SaltstraumenText by Christian Skauge Photos by Stein Johnsen Translated and edited by Arnold Weisz The current grabs you as soon our drive to Saltstraumen, which basi- force of gravity creates enormous as you enter the water. Your cally is a bridge and a few houses, is forces, which the water transforms into nothing but spectacular with snow- one of nature’s many wonders—the first thought, this is going to covered mountains raising out of the malstroem, or whirlpools. The current be a wild one! The adrenalin fjord. sweeping through the sound cre- is flowing as fast in your veins ates the malstroem, which makes the as the currents is flowing past Tidal current water boil. They appear as sudden as The difference between high tide they vanish—huge sucking whirlpools, kelp covered rocks. Diving and low tide in the area around with a diameter of up to 10-12 metres, the strongest malstroem on Saltstraumen can be as much as sucking in water just like a black hole the planet is not for the faint- three metres. The currents force about sucks in surrounding stars. hearted. It is extremely fun 400 cubicmetres of water through A lot of stories circulate about a sound, which is barely 150 metres boats that have vanished in though! wide and three kilometres long. The Saltstraumen. And when you person- The excitement is felt already one the plane as we fly north an hour and half from Oslo, the capital of Norway. -

Marine Current Energy Conversion
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1353 Marine Current Energy Conversion STAFFAN LUNDIN ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9510-7 UPPSALA urn:nbn:se:uu:diva-280763 2016 Dissertation presented at Uppsala University to be publicly examined in Polhemsalen, Ångströmlaboratoriet, Lägerhyddsvägen 1, Uppsala, Wednesday, 4 May 2016 at 13:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor AbuBakr S. Bahaj (University of Southampton). Abstract Lundin, S. 2016. Marine Current Energy Conversion. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1353. 66 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9510-7. Marine currents, i.e. water currents in oceans and rivers, constitute a large renewable energy resource. This thesis presents research done on the subject of marine current energy conversion in a broad sense. A review of the tidal energy resource in Norway is presented, with the conclusion that tidal currents ought to be an interesting option for Norway in terms of renewable energy. The design of marine current energy conversion devices is studied. It is argued that turbine and generator cannot be seen as separate entities but must be designed and optimised as a unit for a given conversion site. The influence of support structure for the turbine blades on the efficiency of the turbine is studied, leading to the conclusion that it may be better to optimise a turbine for a lower flow speed than the maximum speed at the site. -

A Numerical Estimate of the Tidal Energy in the Lofoten Area
A numerical estimate of the tidal energy in the Lofoten area Master thesis in energy Johannes Sandanger Dugstad University of Bergen Department of mathematics and geophysics May 28, 2014 Acknowledgements This master thesis is carried out as a part of the master program in energy at the mathemat- ical and geophysical institute at the University of Bergen. I will especially thank my supervisor Jarle Berntsen for all the help he has given me dur- ing this work. I am very thankful for all the advices he has given me. I appreciate all the discussions we have had when I have come by his office and asked for help. I will also like to thank Helge Avlesen for all his effort by setting up the model that I have used. I will also thank my fellow students for being around during the last 5 years. All the lunches we have had together has been highly appreciated. As I now probably will leave the university, I will miss all the serious and sometimes very unserious conversations we have had during the years. At last I will thank my family for all the support and motivation throughout the work. Johannes S. Dugstad Bergen May 2014 iii Abstract In the following study the energy potential associated with tidal energy will be investigated. A depth integrated model based on the Navier Stokes equations and the continuity equation will be applied to simulate the tides around the Lofoten area in the northern Norway. The model applies a 250 m grid resolution. The only tidal component taken into consideration is the M2-component. -

Maine Tidal In-Stream Energy Conversion (TISEC): Survey and Characterization of Potential Project Sites
Maine Tidal In-Stream Energy Conversion (TISEC): Survey and Characterization of Potential Project Sites Project: EPRI North American Tidal Flow Power Feasibility Demonstration Project Phase: 1 – Project Definition Study Report: EPRI - TP- 003 ME Author: George Hagerman Co-Author: Roger Bedard Date: April 7, 2006 North America Tidal In Stream Energy Conversion Feasibility Study – Site Report - Maine ACKNOWLEDGEMENT The work described in this report was funded by the Maine Technology Initiative (MTI) who, in addition to providing funding for the EPRI Project Team, provided valuable in kind contributions to the project, especially in terms of James Atwell from the MTI Board of Directors Two special acknowledgements are also extended; one to Michael Mayhew of the Maine PUC and the other to Robert Judd, citizen of Lubec Maine and Sacramento, CA, for there tireless work in helping the EPRI team with local knowledge and resources. If, despite our best efforts, errors survive in the pages of this summary report or in our referenced technical reports, the fault rests entirely with the EPRI E2I Global Project Team. And if I inadvertently omitted the names of any persons or organizations that should have been acknowledged, I offer my sincere apologies. Roger Bedard DISCLAIMER OF WARRANTIES AND LIMITATION OF LIABILITIES This document was prepared by the organizations named below as an account of work sponsored or cosponsored by the Electric Power Research Institute Inc. (EPRI). Neither EPRI, any member of EPRI, any cosponsor, the organization (s) -

Uu.Diva-Portal.Org
http://uu.diva-portal.org This is an author produced version of a paper published in Renewable & Sustainable Energy Reviews. This paper has been peer-reviewed but does not include the final publisher proof-corrections or journal pagination. Citation for the published paper: Grabbe, Mårten; Lalander, Emilia; Lundin, Staffan; Leijon, Mats "A review of the tidal current energy resource in Norway" Renewable & Sustainable Energy Reviews, 2009, Vol. 13, Issue 8, pp. 1898-1909 URL: http://dx.doi.org/10.1016/j.rser.2009.01.026 Access to the published version may require subscription. A review of the tidal current energy resource in Norway Marten˚ Grabbe Emilia Lalander Staffan Lundin Mats Leijon The Swedish Centre for Renewable Electric Energy Conversion Division of Electricity, The Angstr˚ om¨ Laboratory Uppsala University P.O. Box 534, SE-751 21 Uppsala, Sweden Telephone: +46 (0)18 471 5843, Fax: +46 (0)18 471 5810 Email: [email protected] Website: http://www.el.angstrom.uu.se/ Abstract As interest in renewable energy sources is steadily on the rise, tidal current energy is receiving more and more attention from politicans, industrialists, and academics. In this article, the conditions for and potential of tidal currents as an energy resource in Norway are reviewed. There having been a relatively small amount of academic work published in this particular field, closely related topics such as the energy situation in Norway in general, the oceanography of the Norwegian coastline, and numerical models of tidal currents in Norwegian waters are also examined. Two published tidal energy resource assessments are reviewed and compared to a desktop study made specifically for this review based on available data in pilot books.