Download Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
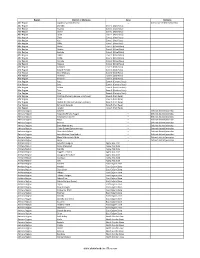
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

REPORT 57 1 September 2021
60 Westminster Rd Oromia Malvern, WR14 4ES, UK upport +44 (0)1684 573722 S [email protected] Group website: oromiasupport.org REPORT 57 1 September 2021 If you want to completely get rid of the fish, you need to dry up the ocean. Fekadu Tessema, Prosperity Party, Oromia branch. Oromia Regional Parliament meeting, Adama, 27 February 2021. This catchphrase of dictators and perpetrators of abuse was used by the Oromia Prosperity Party head to justify ongoing, systematic elimination of Qeerroo, OLA and OLF supporters - to ‘overcome resistance in East and West Wallega, Guji and Borana zones of Oromia’.1 Human rights defenders throughout Oromia, using Qeerroo connections and reporting through the Gadado network, listed over 500 killings between 30 June 2020 and 11 May 2021. Many were previously unrecorded by OSG. The authors report that local kebele and woreda level government officials, working with government soldiers and militias, are ‘assigned to kill innocent Oromo people’. Photographs with the report show bound captives, believed to be relatives of Amanuel Wondimu taken nine days after his public execution on 11 May (OSG Report 56, p.19) and soldiers beating and intimidating young Oromo. Including this report, OSG has recorded 2393 extra-judicial killings of civilians by forces loyal to the Ethiopian government, since October 2018 (excluding Tigray and Afar Regions). 1612 were Oromo and 924 of them were killed in West Oromia. Killings in this report were particularly prevalent September to December 2020. When Federal forces, Eritrean soldiers and Amhara Region militia became more engaged in Tigray, the rate of killings in Oromia dropped. -

Addis Ababa University College of Education and Behavioral Studies Department of Educational Planning and Management
ADDIS ABABA UNIVERSITY COLLEGE OF EDUCATION AND BEHAVIORAL STUDIES DEPARTMENT OF EDUCATIONAL PLANNING AND MANAGEMENT PRACTICES AND CHALLENGES OF IMPLEMENTING ACTIVE LEARNING METHODOLOGY IN THE SECONDARY SCHOOLS OF WEST WOLLEGA ZONE, OROMIA NATIONAL REGIONAL STATE BY MELKAMU HAILU ADDIS ABABA, ETHIOPIA OCTOBER, 2018 ADDIS ABABA UNIVERSITY COLLEGE OF EDUCATION AND BEHAVIORAL STUDIES DEPARTMENT OF EDUCATIONAL PLANNING AND MANAGEMENT This is to certify that the thesis prepared by Melkamu Hailu entitled study on the Practices and challenges of implementing active learning methodology in the secondary school of west wollega zone: Oromia national regional state in partial fulfillment of the requirement for the degree of master of education in school leadership complies with the regulation of the university and meets the accepted standards with respect to originality and quality Signed by examining committee Advisor Demis Zergaw (PHD) ______________ ________________ Signature Date ______________ ________________ ________________ Internal examiner Signature Date ______________ ________________ ________________ External examiner Signature Date ______________ ______________ ______________ Chairman, Department Graduate Committee Signature Date ii DECLARATION I, the under signed, declare that this thesis is my original work and has not been presented in any other university, and that all sources of materials used for this thesis have been duly acknowledged. Name: Melkamu Hailu _____________ __________________ Signature Date This thesis has been submitted for examination with my approval as university advisor. Name: Demis Zergaw (PhD) ____________________ ____________________ Signature Date iii Acknowledgements Above all, I would like to thank God, the most gracious and the most merciful, for the guidance, compassion and mercy which He has bestowed upon me throughout my entire life and n particular while working on this thesis. -

The Role of Multi-Purpose Cooperatives in the Economic Development in Ethiopia, the Case of Lalo-Assabi District (West Wollega Zone, Oromia Regional State)
Research on Humanities and Social Sciences www.iiste.org ISSN 2224-5766 (Paper) ISSN 2225-0484 (Online) Vol.9, No.21, 2019 The Role of Multi-Purpose Cooperatives in the Economic Development in Ethiopia, The Case of Lalo-Assabi District (West Wollega Zone, Oromia Regional State) Desalegn Fekadu Etefa Ambo University: Institute of Cooperatives and Development Studies, Department of Cooperative Abstract The objective of this study is to show the overall development trends and the challenges encountering the multi- purpose cooperatives (MPCs). To do so, research methodology employed was descriptive, which involved both qualitative and quantitative methods. Data were collected from both primary and secondary sources. The primary data were collected from the sample through cross-sectional survey from 210 respondents. Besides, 16 key informants and three focus group discussions (FGDs) were considered in the assessment. The instruments used were questionnaires, FGDs, in depth interviews, observation and document review. Both probability and non- probability sampling techniques were applied. Data analyses were done using descriptive statistics. The result of the study shows the average rate of growth of members in sample MPCs is 19% as calculated from 2016- 2018 and 79.3% from their formation period. The capital of the MPCs increased from 305,081 to 9.3 million and their profit was nearly 1.5 million ETB. Of the multifaceted services they provide, marketing agricultural inputs accounts for the great proportions. In general, the sample MPCs are contributing somewhat in the economic development. However, their performance is not as expected due to major problems identified: lack of professional managers, devoted management committees, limited capital base; weak horizontal and vertical linkages, low members’ participation, insufficient dividend, lack of diversified activities; limited awareness, inadequate infrastructure, low stakeholders’ participation, lack of adequate credit and necessary technical supports are among the hitches to be tackled. -

Electromagnetic Radiation (EMR) Clashes with Honeybees (Retracted) Sainudeen Pattazhy 58
OPEN ACCESS Journal of Entomology and Nematology July 2019 ISSN 2006-9855 DOI: 10.5897/JEN www.academicjournals.org About JEN Journal of Entomology and Nematology (JEN) is an open access journal that provides rapid publication (monthly) of articles in all areas of the subject such as applications of entomology in solving crimes, taxonomy and control of insects and arachnids, changes in the spectrum of mosquito-borne diseases etc. The Journal welcomes the submission of manuscripts that meet the general criteria of significance and scientific excellence. Papers will be published shortly after acceptance. All articles published in JEN are peer-reviewed. Contact Us Editorial Office: [email protected] Help Desk: [email protected] Website: http://www.academicjournals.org/journal/JEN Submit manuscript online http://ms.academicjournals.me/ Editors Dr. Abir Al-Nasser Dr. Mukesh K. Dhillon Department of Biology International Crops Research Institute for the Umm Al Qura University Semi-Arid Tropics (ICRISAT) Saudi Arabia. GT-Biotechnology Andhra Pradesh, India. Editorial Board Members Prof. Liande Wang Dr. Sam Manohar Das Faculty of Plant Protection Dept. of PG studies and Research Centre in Fujian Agriculture and Forestry University Zoology Fuzhou, Scott Christian College (Autonomous) China. India Dr. Raul Neghina Dr. Leonardo Gomes Victor Babes University of Medicine and Pharmacy Universidade Estadual Paulista “Júlio de Mesquita Timisoara, Filho” (UNESP) Romania. Depto de Biologia Rio Claro, Prof. Fukai Bao Brazil. Kunming Medical University Kunming, Dr. Mahloro Hope Serepa-Dlamini China. Biotechnology and Food Technology, University of Johannesburg, Dr. Anil Kumar Dubey South Africa. Department of Entomology National Taiwan University Taipei, Dr. Bindiya Sachdev Taiwan. -

Addis Ababa University, College of Health Sciences, School of Public Health
Addis Ababa University, College of Health Sciences, School of Public Health Ethiopia Field Epidemiology Training Program (EFELTP) Compiled Body of Works in Field Epidemiology By: Natnael Teferi Dejene Submitted to the School of Graduate Studies of Addis Ababa University in Partial Fulfillment for the Degree of Master of Public Health in Field Epidemiology June, 2019 Addis Ababa | P a g e 1.Page Addis Ababa University, College of Health Sciences, School of Public Health Ethiopia Field Epidemiology Training Program (EFELTP) Compiled Body of Works in Field Epidemiology By: Natnael Teferi Dejene Submitted to the School of Graduate Studies of Addis Ababa University in Partial Fulfillment for the Degree of Master of Public Health in Field Epidemiology Advisors: Dr. Negussie Deyessa Dr. Girma Taye ii | P a g e [email protected] AAU/SPH_EFE L TP ADDIS ABABA UNIVERSITY School of Graduate Studies Compiled Body of Works in Field Epidemiology By: Natnael Teferi Dejene Ethiopia Field Epidemiology Training Program (EFELTP) School of Public Health, College of Health Sciences Addis Ababa University Approval by Examining Board ___________________ ___________________ Chairman, School Graduate Committee ___________________ ___________________ Advisor ___________________ ___________________ Examiner ___________________ ___________________ Examiner iii | P a g e [email protected] AAU/SPH_EFE L TP Acknowledgement I would like to express my deepest gratitude to Almighty God for his invaluable and never-ending support and beneficent me all the way of life. There are many contributors those were directly or indirectly participated in the fruitfulness of this document starting from individuals to institutions and organizations. First I want to thank Dr. Negussie Deyessa and Dr Girma Taye for their constant support and wealth of helpful suggestions and advice. -

Waketola Getachew.Pdf
ADAMA SCIENCE AND TECHNOLOGY UNIVERSITY THE SCHOOL OF HUMANITIES AND LAW DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES DETERMINANTS OF LAND DEGRADATION, IN NEJO WOREDA, WESTERN ETHIOPIA BY WAKETOLA GETACHEW MA THESIS SUBMITTED TO SCHOOL OF HUMANITIES AND LAW DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES AUGUST, 2017 ADAMA, ETHIOPIA. i ADAMA SCIENCE AND TECHNOLOGY UNIVERSITY THE SCHOOL OF HUMANITIES AND LAW DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES DETERMINANTS OF LAND DEGRADATION, IN NEJO WOREDA, WESTERN ETHIOPIA BY WAKETOLA GETACHEW ADVISOR DR. TSETADRGACHEW LEGESSE MA THESIS SUBMITTED TO SCHOOL OF HUMANITIES AND LAW DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES INPARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREEOF MASTER OF ARTS IN GEOGRAPHY AND ENVIRONMENTAL STUDIES AUGUST, 2017 ADAMA, ETHIOPIA. i Declaration I declare that this thesis is my original work and that all sources of materials used for this thesis have been duly acknowledged. I solemnly declare that this thesis has never been presented to any other institution anywhere for the award of any academic degree. This thesis has been submitted in partial fulfillment of the requirement for M.A. Degree in Geography and Environmental Studies at Adama Science and Technology University. Name: Waketola Getachew Signature_____________ Date_________________ i Acknowledgment Above all, I would like to thank the Almighty God for his help in all aspects to finalize my study. I would like to express my sincere gratitude to my advisor Dr. Tsetadrgachew Legesse for his invaluable advice, comments, encouragement and assistance throughout the thesis work. I have no words to thank and appreciate Ato Girma Wadajo and Ato Tesfa Mosisa for their continuous support morally and materially. -

Ethiopia Emergency Type: Complex Reporting Period: 1-30 April 2019
HEALTH CLUSTER BULLETIN #3 Getting to work. April 2019 Photo: MCMDO Ethiopia Emergency type: Complex Reporting period: 1-30 April 2019 6.0 MILLION 2.4 M IDP 2.4 M HOST IN NEED TARGETED TARGETED 445 WOREDAS HIGHLIGHTS HEALTH SECTOR The return process already started in various IDP HEALTH CLUSTER locations, including Gedeo, West Guji, Assosa, 19 IMPLEMENTING PARTNERS Kamashi, East Wellega, West Wellega, East Hararge, Borena, Fafan and Dawa. Many IDP have MEDICINES DELIVERED TO HEALTH FACILITIES/PARTNERS asked for assurances on their safety and security in areas of return and origin, and continued 21 ASSORTED MEDICAL KITS humanitarian assistance. HEALTH CLUSTER ACTIVITIES FMOH/EPHI declared AWD outbreak in northern Amhara, with one case confirmed as vibrio cholerae. So far 182 cases and 12 deaths were 38,352 OPD CONSULTATIONS reported; Telemt woreda 111 cases and 8 deaths, Abergelie woreda 68 cases and 3 deaths, and Beyeda woreda 3 cases and 1 death. VACCINATION The Health Cluster received $6.2M from the first 779,126 VACCINATED AGAINST MEASLES standard allocation of the EHF. Out of this, $2.45M was assigned to emergency health pipeline to EWARS restock assorted medical and reproductive health kits. The rest was assigned to frontline projects 1 CONFIRMED AWD OUTBREAK targeting woredas hosting IDP and returnees in Assosa, Kamashi, West Wellega, East Wellega, West FUNDING $US Hararge, East Hararge, West Guji, Gedeo, and 143 M REQUESTED Borena. Also 10 woredas in Somali region were 11.3 M 7.9% FUNDED prioritized by the sub-national Cluster. 131.7 M GAP PAGE 1 Situation update In April, the Ministry of Peace and NDRMC launched the government’s strategic plan to address internal displacement in Ethiopia. -

National Wash Program Selected Woredas and Small Towns
National WaSH Program Selected Woredas and Small Towns 1. Oromiya Region No Oromiya Woredas No Oromiya Woredas 41 Dugda 83 Ana Sora No Oromiya Woredas 42 Liban Chukala 84 Seba Boru 1 Digalu Tijo 43 Lume 85 Uraga 2 Gololcha 44 Bora 86 Odo Shakkiso 3 Jeju 45 Gudeya Bila 87 Liban 4 Limu Bilbilo 46 Guto Gida 88 Fentale 5 Tiyo 47 Sasiga 89 Babile 6 Zuway Dugda 48 Sibu Sire 90 Chinaksen 7 Adaba 49 Wayu Tuka 91 Qumbi 8 Gedeb Asasa 50 Seyo Nole 92 Guba Korcha 9 Agarfa 51 Nedjo 93 Chiro 10 Dawe Serer 52 Haru 94 Chewaka 11 Dinsho 53 Dale Sedi 95 Hurum 12 Gindhir 54 Dale Wabera 96 Darimu 13 Goro 55 Gawo Kebe 97 Dedo 14 Rayitu 56 Lalo Kile 98 Sokoru 15 Sinana 57 Seyo 99 Seka Chokorsa 16 Bedeno 58 Yubdo 100 Limu Seka 17 Gorogutu 59 Ayira 101 Ilu 18 Girawa 60 Guliso 102 Sodo Dachi 19 Kurfa Chale 61 Sude 103 Weliso 20 Kombolcha 62 Doddota 104 Ameya 21 Haromaya 63 Inkolo Wabe 105 Seden Soddo 22 Kersa 64 Robe 106 Nonno 23 Fedis 65 Lege Hidha 107 Dera 24 Boke 66 Sewena 108 Girar Jarso 25 Darulabu 67 G/Dhamole 109 Yaya Gulele 26 Doba 68 Siraro 110 Kuyu 27 Oda Bultum 69 Arsi Negele 111 Wuchale 28 Tulo 70 Kofele 112 Sululta 29 Hawi Gudina 71 Shashemene 113 Mulo 30 Burqa Dimtu 72 Kokosa 114 Sebeta Hawas 31 Becho 73 Akaki 115 Wolmera 32 Bedele 74 Teltale 116 Berek 33 Bilo Nopha 75 Dhas 117 Dendi 34 Didu 76 Yabello 118 Meta Robi 35 Metu 77 Arero 119 Abuna Ginde Beret 36 Yayu 78 Bule Hora 120 Ambo 37 Dorani 79 Gelana 121 Ilu Gelan 38 Adea 80 Moyale 122 Jibat 39 Adama 81 Hambela Wamana 123 Mida Kegn 40 AT/Jido Kombolcha 82 Kercha 124 Toke Kutaye National WaSH -

Prevalence and Identification of Ixodide Ticks in Cattle in Lalo Assabi District, West Wollega Zone, West Oromia, Ethiopia
Open Access Journal of Veterinary Science & Research ISSN: 2474-9222 Prevalence and Identification of Ixodide Ticks in Cattle in Lalo Assabi District, West Wollega Zone, West Oromia, Ethiopia Kebede A*, Lemmi E and Dugassa J Research Article School of Veterinary Medicine, College of Medical and Health Science, Wollega Volume 3 Issue 3 University, Ethiopia Received Date: August 07, 2018 Published Date: September 07, 2018 *Corresponding author: Abriham Kebede, School of Veterinary Medicine, College of Medical and Health Science, Wollega University, P.O. Box, 395, Nekemte, Ethiopia, Tel: 251-917-095-077; Email: [email protected] Abstract Ticks are important parasite of cattle that cause huge economic loss at private and national level. Therefore, the cross sectional study was conducted from October 2016 to June 2017 in Lalo Assabi district of West Wollega Zone. The aims of the study were to determine the prevalence of tick infestation and to identify their predilection site, possible risk factors and distribution in the rural and urban area of Lalo Assabi districts. Peasant Associations (PAs), sex, age, body conditions of animals and ticks genera were major factors involved in the study. A systematic random sampling study design was followed to collect samples. From the total of 384 cattle, 265 (69%) cattle were found to be infested with tick. Out of five peasant associations examined, highest prevalence (80.4%) was recorded at Barko Daleti Peasant Associations. Highest prevalence (82%) was observed in male than in female animals (59%). Relatively highest prevalence was recorded in adult animals (70.7%) as compared with young and old age animals. -

Labsii 198 Bara 2008.Pdf
Waggaa 25 ffaa..... Lak. ....1 /2008 Finfinnee,............. Waxabajii 2, 2008 ፳5ኛ ዓመት........ ቁጥር.....1/፪ሺ8 ፊንፊኔ፣.................. c’@ 2 ቀን 2ሺ8 25thyear ........... No. ....... 1/2017 Finfine,......................... June ,9 2016 MAGALATA OROMIYAA L µ E p ‰ Z Më ¦ MEGELETA OROMIA Gatiin Tokkoo ........... Qarshii 13.20 To’annoo Caffee Mootummaa Naannoo Lak. S. Poostaa ......... 21383-1000 Oromiyaatiin Kan Bahe ¦¿«è ”¶ ........................ ብር 13.20 £Þ.Q.e¼Y ...............21383-1000 Unit Price ....................... Birr 13.20 l‰ZMë¦ wN?^© ŒGF”í L¿·Rr uÚô *aT>Á ºmf}r £’» P.O.Box ....................... 21383-1000 Proclamation No. 198/2016 Labsii Lak. 198/2008 አዋጅ ቁጥር )(8/፪ሺ8 Labsii Baajata Daabalataa Hojiiw- Supplementary Budget Proclama- wan Mootummaa Naannoo Oromi- K*aT>Á ¡ML© S”ÓYƒ Y^ዎ ‹ ¾¨Ë tion For Oromia Regional State 1 yaati Labsame......................... Fuula 1 ¾}ÚTሪ u˃ ›ªÏ......................... ገጽ Services.................................... page 1 Labsii Baajata Daabalataa Hojiiwwan K*aT>Á ¡ML© S”ÓYƒ Y^ዎ ‹ Supplementary Budget Proclama- Mootummaa Naannoo Oromiyaati ¾¨Ë ¾}ÚTሪ u˃ ›ªÏ tion For Oromia Regional State Labsame Services ለ2ሺ8 u˃ ¯Sƒ u*aT>Á ¡MM WHEREAS, it is necessary to adopt Bara baajataa 2008 tti hojiiwwanii fi ¨<eØ KT>Ÿ“¨’<ƒ Y^ ‹“ ›ÑMግKA„‹ and implement the supplement tajaajilawwan Naannoo Oromiyaa budgetary appropriation which keessatti adeemsifamaniif baajatni ¾T>ÁeðMѨ<” }ÚTሪ u˃ ›êÉq are required for the purposes and dabalata barbaachisaa ta'e raggaa- Y^ Là TªM ›eðLÑ> uSJ’<' services of Oromia Regional State sifamee hojiirra oolchuun barbaa- For the 2008 Ethiopian fiscal year, }hhKA u¨×¨< ¾*aT>Á wN?^© chiisaa waan ta'eef, NOW, THEREFORE, in accordance ¡ML© S”ÓYƒ QÑ S”ÓYƒ with Article 49 (3)(a) of the Revised Akkaataa Heera Mootummaa Naan- ›ªÏ lØ` ፵፮/፲፱፻፺፬ ›”kê ፵፱ (፫) Constitution of the National Regional noo Oromiyaa Fooyya’ee Bahe Lab- State of Oromia it is hereby proclaimed (G) SW[ƒ ¾T>Ÿ}K¨< ¨<ÍDM:: sii Lak. -

Adama Science and Technology University
ADAMA SCIENCE AND TECHNOLOGY UNIVERSITY SCHOOL OF HUMANITIES AND LAW DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES CAUSES OF DEFORESTATION IN NEJO WOREDA, WESTERN ETHIOPIA BY: GIRMA SHUMETA MA THESIS SUBMITTED TO DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES ADAMA SCIENCE AND TECHNOLOGY UNIVERSITY AUGUST, 2017 ADAMA, ETHIOPIA ADAMA SCIENCE AND TECHNOLOGY UNIVERSITY SCHOOL OF GRADUATE STUDIES As Thesis advisor, I hereby certify that I have read and evaluated this Thesis prepared, under my guidance, by Girma Shumeta Abdeta entitled “Determinants of Deforestation in Nejo Wereda, Western Ethiopia” I recommend that it be submitted as fulfillment of the Thesis requirement. MR.GEZMU HUNDE ___________________ ______________ Advisor Signature Date BY GIRMA SHUMETA ABDETA APPROVED BY THE BOARD OF EXAMINERS As member of the Board of Examiners of the MA Thesis Open Defense Examination, we certify that we have read, evaluated the Thesis prepared by Girma Shumeta Abdeta and examined the candidate. We recommended that the Thesis be accepted, as fulfilling the Thesis requirement for the Degree of Master of Art in Geography and Environmental Studies. Name ____________________ _____________________ ______________ Chairman Signature Date ______________________ _____________________ ______________ Advisor Signature Date ______________________ _____________________ ______________ External Examiner Signature Date ______________________ _____________________ ______________ Internal Examiner Signature Date ____________________ _________________ _______________ DGC Chairman Signature Date DECLARATION First, I declare that this thesis is my original work and that the sources of all the material used in thesis have been duly acknowledged. This thesis was submitted in partial fulfillment of the requirements for MA degree at Adama Science and Technology University. It has also been deposited at the University Library to be made available for borrowers in accordance with the rules of the library.