Prefrontal Cortex in Humans and Apes: a Comparative Study of Area 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PASA 2005 Final Report.Pdf
PAN AFRICAN SANCTUARY ALLIANCE 2005 MANAGEMENT WORKSHOP REPORT 4-8 June 2005 Mount Kenya Safari Lodge, Nanyuki, Kenya Hosted by Pan African Sanctuary Alliance / Sweetwaters Chimpanzee Sanctuary Photos provided by Tacugama Chimpanzee Sanctuary – Sierra Leone (cover), PASA member sanctuaries, and Doug Cress. A contribution of the World Conservation Union, Species Survival Commission, Conservation Breeding Specialist Group (CBSG) and Primate Specialist Group (PSG). © Copyright 2005 by CBSG IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Prepared by participants in the PASA 2005 Management Workshop, Mount Kenya, Kenya, 4th – 8th June 2005 W. Mills, D. Cress, & N. Rosen (Editors). Conservation Breeding Specialist Group (SSC/IUCN). 2005. Pan African Sanctuary Alliance (PASA) 2005 Workshop Report. Additional copies of the Pan African Sanctuary Alliance (PASA) 2005 Workshop Report can be ordered through the IUCN/SSC Conservation -
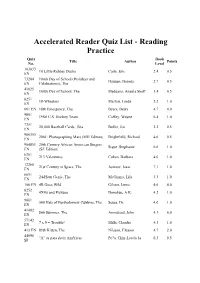
Accelerated Reader Quiz List - Reading Practice Quiz Book Title Author Points No
Accelerated Reader Quiz List - Reading Practice Quiz Book Title Author Points No. Level 103833 10 Little Rubber Ducks Carle, Eric 2.4 0.5 EN 73204 100th Day of School (Holidays and Haugen, Brenda 2.7 0.5 EN Celebrations), The 41025 100th Day of School, The Medearis, Angela Shelf 1.4 0.5 EN 8251 18-Wheelers Maifair, Linda 5.2 1.0 EN 661 EN 18th Emergency, The Byars, Betsy 4.7 4.0 9801 1980 U.S. Hockey Team Coffey, Wayne 6.4 1.0 EN 7351 20,000 Baseball Cards...Sea Buller, Jon 2.5 0.5 EN 900355 2061: Photographing Mars (MH Edition) Brightfield, Richard 4.6 0.5 EN 904851 20th Century African American Singers Sigue, Stephanie 6.6 1.0 EN (SF Edition) 6201 213 Valentines Cohen, Barbara 4.0 1.0 EN 12260 21st Century in Space, The Asimov, Isaac 7.1 1.0 EN 6651 24-Hour Genie, The McGinnis, Lila 3.3 1.0 EN 166 EN 4B Goes Wild Gilson, Jamie 4.6 4.0 8252 4X4's and Pickups Donahue, A.K. 4.2 1.0 EN 9001 500 Hats of Bartholomew Cubbins, The Seuss, Dr. 4.0 1.0 EN 41482 $66 Summer, The Armistead, John 4.3 6.0 EN 57142 7 x 9 = Trouble! Mills, Claudia 4.3 1.0 EN 413 EN 89th Kitten, The Nilsson, Eleanor 4.7 2.0 44096 "A" es para decir Am?ricas Pe?a, Chin-Lee/de la 6.3 0.5 SP Accelerated Reader Quiz List - Reading Practice Quiz Book Title Author Points No. -

Connectivity of BA46 Involvement in the Executive Control of Language
Alfredo Ardila, Byron Bernal and Monica Rosselli Psicothema 2016, Vol. 28, No. 1, 26-31 ISSN 0214 - 9915 CODEN PSOTEG Copyright © 2016 Psicothema doi: 10.7334/psicothema2015.174 www.psicothema.com Connectivity of BA46 involvement in the executive control of language Alfredo Ardila1, Byron Bernal2 and Monica Rosselli3 1 Florida International University, 2 Miami Children’s Hospital and 3 Florida Atlantic University Abstract Resumen Background: Understanding the functions of different brain areas has Estudio de la conectividad del AB46 en el control ejecutivo del lenguaje. represented a major endeavor of contemporary neurosciences. Modern Antecedentes: la comprensión de las funciones de diferentes áreas neuroimaging developments suggest cognitive functions are associated cerebrales representa una de las mayores empresas de las neurociencias with networks rather than with specifi c areas. Objectives. The purpose contemporáneas. Los estudios contemporáneos con neuroimágenes of this paper was to analyze the connectivity of Brodmann area (BA) 46 sugieren que las funciones cognitivas se asocian con redes más que con (anterior middle frontal gyrus) in relation to language. Methods: A meta- áreas específi cas. El propósito de este estudio fue analizar la conectividad analysis was conducted to assess the language network in which BA46 is del área de Brodmann 46 (BA46) (circunvolución frontal media anterior) involved. The DataBase of Brainmap was used; 19 papers corresponding con relación al lenguaje. Método: se llevó a cabo un meta-análisis para to 60 experimental conditions with a total of 245 subjects were included. determinar el circuito o red lingüística en la cual participa BA46. Se utilizó Results: Our results suggest the core network of BA46. -

Dorsolateral Prefrontal Cortex-Based Control with an Implanted Brain–Computer Interface
www.nature.com/scientificreports OPEN Dorsolateral prefrontal cortex‑based control with an implanted brain–computer interface Sacha Leinders 1, Mariska J. Vansteensel 1, Mariana P. Branco 1, Zac V. Freudenburg1, Elmar G. M. Pels1, Benny Van der Vijgh1, Martine J. E. Van Zandvoort2, Nicolas F. Ramsey1* & Erik J. Aarnoutse 1 The objective of this study was to test the feasibility of using the dorsolateral prefrontal cortex as a signal source for brain–computer interface control in people with severe motor impairment. We implanted two individuals with locked‑in syndrome with a chronic brain–computer interface designed to restore independent communication. The implanted system (Utrecht NeuroProsthesis) included electrode strips placed subdurally over the dorsolateral prefrontal cortex. In both participants, counting backwards activated the dorsolateral prefrontal cortex consistently over the course of 47 and 22months, respectively. Moreover, both participants were able to use this signal to control a cursor in one dimension, with average accuracy scores of 78 ± 9% (standard deviation) and 71 ± 11% (chance level: 50%), respectively. Brain– computer interface control based on dorsolateral prefrontal cortex activity is feasible in people with locked‑in syndrome and may become of relevance for those unable to use sensorimotor signals for control. Locked-in syndrome (LIS) is characterized by intact cognition and (almost) complete loss of voluntary movement1. Brain–computer interface (BCI) systems allow computer control with brain activity and can there- fore provide an alternative communication channel to people with LIS. BCI systems depending on self-initiated modulation of brain activity generally measure from sensorimotor areas, e.g.2–9. However, not all people with LIS may be able to reliably modulate sensorimotor activity, due to for instance atrophy secondary to neurode- generative disease10,11, damage afer stroke or injury, or atypical neural activity 12. -

Folia De Reis, a Brazilian Spiritual Journey Through Song Welson Alves Tremura
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2004 With an Open Heart: Folia De Reis, a Brazilian Spiritual Journey Through Song Welson Alves Tremura Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY SCHOOL OF MUSIC WITH AN OPEN HEART: FOLIA DE REIS, A BRAZILIAN SPIRITUAL JOURNEY THROUGH SONG By WELSON ALVES TREMURA A Dissertation submitted to the School of Music in partial fulfillment of the Requirements for the degree of Doctor of Philosophy Degree Awarded: Spring Semester, 2004 Copyright 2004 Welson Alves Tremura All Rights Reserved The members of the Committee approve the dissertation of Welson Alves Tremura defended on April 5, 2004. _____________________________ Dale A. Olsen Professor Directing Dissertation _____________________________ Anthony Oliver-Smith Outside Committee Member _____________________________ Michael B. Bakan Committee Member _____________________________ Larry Crook Committee Member The Office of Graduate Studies has verified and approved the above named committee members. ii The folia de reis high-pitched voices singing in the distance are memories of a childhood of music and celebrations that go back to the early 1970s when playing soccer in a field not larger than a basketball court or flying a kite were the highest point of most children in that part of Brazil. The folia groups could be heard in the distance with the tala or high pitch voice singing the last part of the refrain. These sounds could typically be heard echoing throughout the surrounding neighborhoods of Olímpia, São Paulo during the second half of the month of December and early part of January. -

ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT
ICC-01/09-02/11-67-Anx3 21-04-2011 1/84 EO PT No. ICC-01/09-02/11 21-4-11 ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT A PROGRESS REPORT TO THE HON. ATTORNEY-GENERAL BY THE TEAM ON UPDATE OF POST ELECTION VIOLENCE RELATED CASES IN WESTERN, NYANZA, CENTRAL, RIFT-VALLEY, EASTERN, COAST AND NAIROBI PROVINCES MARCH, 2011 NAIROBI ICC-01/09-02/11-67-Anx3 21-04-2011 3/84 EO PT TABLE OF CONTENTS CHAPTER SUBJECT PAGE TRANSMITTAL LETTER IV 1. INTRODUCTION 1 2. GENDER BASED VIOLENCE CASES 7 3. WESTERN PROVINCE 24 3. RIFT VALLEY PROVINCE 30 4. NYANZA PROVINCE 47 5. COAST PROVINCE 62 6. NAIROBI PROVINCE 66 7. CENTRAL PROVINCE 69 8. STATISTICAL ANALYSIS 70 9. CONCLUSION 73 10. APPENDICES ICC-01/09-02/11-67-Anx3 21-04-2011 4/84 EO PT APPENDIX (NO.) LIST OF APPENDICES APP. 1A - Memo from CPP to Hon. Attorney General APP.1B - Memo from CPP to Hon. Attorney General APP.1C - Update on 2007 Post Election Violence offences As at 4th March, 2010 (police commissioner’s report) APP. 1D - Update by Taskforce on Gender Based Violence Cases (police commissioner’s report) APP. 2 - Memo to Solicitor- General from CPP APP. 3 - Letter from PCIO Western APP. 4 - Letter from PCIO Rift Valley APP.5 - Cases Pending Under Investigations in Rift Valley on special interest cases APP.6 - Cases where suspects are known in Rift Valley but have not been arrested APP.7 - Letter from PCIO Nyanza APP.8 - Letter from PCIO Coast APP.9 - Letter from PCIO Nairobi APP.10 - Correspondences from the team ICC-01/09-02/11-67-Anx3 21-04-2011 5/84 EO PT The Hon. -

Unraveling the Evolutionary History of Orangutans (Genus: Pongo)- the Impact of Environmental Processes and the Genomic Basis of Adaptation
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Unraveling the evolutionary history of Orangutans (genus: Pongo)- the impact of environmental processes and the genomic basis of adaptation Mattle-Greminger, Maja Patricia Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-121397 Dissertation Published Version Originally published at: Mattle-Greminger, Maja Patricia. Unraveling the evolutionary history of Orangutans (genus: Pongo)- the impact of environmental processes and the genomic basis of adaptation. 2015, University of Zurich, Faculty of Science. Unraveling the Evolutionary History of Orangutans (genus: Pongo) — The Impact of Environmental Processes and the Genomic Basis of Adaptation Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch‐naturwissenschaftlichen Fakultät der Universität Zürich von Maja Patricia Mattle‐Greminger von Richterswil (ZH) Promotionskomitee Prof. Dr. Carel van Schaik (Vorsitz) PD Dr. Michael Krützen (Leitung der Dissertation) Dr. Maria Anisimova Zürich, 2015 To my family Table of Contents Table of Contents ........................................................................................................ 1 Summary ..................................................................................................................... 3 Zusammenfassung ..................................................................................................... -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Duncan Public Library Board of Directors Meeting Minutes June 23, 2020 Location: Duncan Public Library
Subject: Library Board Meeting Date: August 25, 2020 Time: 9:30 am Place: Zoom Meeting 1. Call to Order with flag salute and prayer. 2. Read minutes from July 28, 2020, meeting. Approval. 3. Presentation of library statistics for June. 4. Presentation of library claims for June. Approval. 5. Director’s report a. Summer reading program b. Genealogy Library c. StoryWalk d. Annual report to ODL e. Sept. Library Card Month f. DALC grant for Citizenship Corner g. After-school snack program 6. Consider a list of withdrawn items. Library staff recommends the listed books be declared surplus and be donated to the Friends of the Library for resale, and the funds be used to support the library. 7. Consider approving creation of a Student Library Card and addition of policy to policy manual. 8. Old Business 9. New Business 10. Comments a. By the library staff b. By the library board c. By the public 11. Adjourn Duncan Public Library Claims for July 1 through 31, 2020 Submitted to Library Board, August 25, 2020 01-11-521400 Materials & Supplies 20-1879 Demco......................................................................................................................... $94.94 Zigzag shelf, children’s 20-2059 Quill .......................................................................................................................... $589.93 Tissue, roll holder, paper, soap 01-11-522800 Phone/Internet 20-2222 AT&T ........................................................................................................................... $41.38 -

Williams Dissertation
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Don't Show A Hyena How Well You Can Bite: Performance, Race and the Animal Subaltern in Eastern Africa Permalink https://escholarship.org/uc/item/0jf3488f Author Williams, Joshua Publication Date 2017 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Don’t Show A Hyena How Well You Can Bite: Performance, Race and the Animal Subaltern in Eastern Africa by Joshua Drew Montgomery Williams A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Performance Studies and the Designated Emphasis in Critical Theory in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Catherine Cole, Chair Professor Donna Jones Professor Samera Esmeir Professor Brandi Wilkins Catanese Spring 2017 Abstract Don’t Show A Hyena How Well You Can Bite: Performance, Race and the Animal Subaltern in Eastern Africa by Joshua Drew Montgomery Williams Doctor of Philosophy in Performance Studies Designated Emphasis in Critical Theory University of California, Berkeley Professor Catherine Cole, Chair This dissertation explores the mutual imbrication of race and animality in Kenyan and Tanzanian politics and performance from the 1910s through to the 1990s. It is a cultural history of the non- human under conditions of colonial governmentality and its afterlives. I argue that animal bodies, both actual and figural, were central to the cultural and -

Tanzania Chimpanzee Conservation Action Plan 2018-2023
Tanzania Chimpanzee Conservation Action Plan 2018-2023 This plan is written in collaboration with various institutions that have interest and are working tirelessly in conserving chimpanzees in Tanzania. Editorial list i. Dr. Edward Kohi ii. Dr. Julius Keyyu iii. Dr. Alexander Lobora iv. Ms. Asanterabi Kweka v. Dr. Iddi Lipembe vi. Dr. Shadrack Kamenya vii. Dr. Lilian Pintea viii. Dr. Deus Mjungu ix. Dr. Nick Salafsky x. Dr. Flora Magige xi. Dr. Alex Piel xii. Ms. Kay Kagaruki xiii. Ms. Blanka Tengia xiv. Mr. Emmanuel Mtiti Published by: Tanzania Wildlife Research Institute (TAWIRI) Citation: TAWIRI (2018) Tanzania Chimpanzee Conservation Action Plan 2018-2023 TAWIRI Contact: [email protected] Cover page photo: Chimpanzee in Mahale National Park, photo by Simula Maijo Peres ISBN: 978-9987-9567-53 i Acknowledgements On behalf of the Ministry of Natural Resource and Tourism (MNRT), Wildlife Division (WD), Tanzania National Parks (TANAPA), and Tanzania Wildlife Authority (TAWA), the Tanzania Wildlife Research Institute (TAWIRI) wishes to express its gratitude to organizations and individuals who contributed to the development of this plan. We acknowledge the financial support from the U.S. Agency for International Development (USAID) and the U.S Fish and Wildlife Service (USFWS) for the planning process. Special thanks are extended to the Jane Goodall Institute (JGI) and Kyoto University for their long-term chimpanzee research in the country that has enhanced our understanding of the species behaviour, biology and ecology, thereby greatly contributing to the development process of this conservation action plan. TAWIRI also wishes to acknowledge contributions by Conservation Breeding Specialist Group - Species Survival Commission of the International Union for Conservation of Nature (IUCN), Evaluation and Research Technologies for Health, Inc. -

Healthy and Abnormal Development of the Prefrontal Cortex
Developmental Neurorehabilitation, October 2009; 12(5): 279–297 Healthy and abnormal development of the prefrontal cortex MEGAN SPENCER-SMITH1,2 & VICKI ANDERSON1,2,3 1Murdoch Childrens Research Institute, Melbourne, Australia, 2University of Melbourne, Melbourne, Australia, and 3Royal Children’s Hospital, Melbourne, Australia (Received 8 October 2008; accepted 3 June 2009) Abstract Background: While many children with brain conditions present with cognitive, behavioural, emotional, academic and social impairments, other children recover with seemingly few impairments. Animal studies and preliminary child studies have identified timing of brain lesion as a key predictor in determining functional outcome following early brain lesions. Review: This research suggests that knowledge of healthy developmental processes in brain structure and function is essential for better understanding functional recovery and outcome in children with brain lesions. This review paper aims to equip researchers with current knowledge of key principles of developmental processes in brain structure and function. Timetables for development of the prefrontal cortex (PFC), a brain region particularly vulnerable to lesions due to its protracted developmental course, are examined. In addition, timetables for development of executive skills, which emerge in childhood and have a prolonged developmental course that parallels development of the PFC, are also discussed. Conclusions: Equipped with this knowledge, researchers are now in a better position to understand functional