Fall 01 for Pdf.Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Composition of the Wood Oils of Calocedrus Macrolepis, Calocedrus
American Journal of Essential Oils and Natural Products 2013; 1 (1): 28-33 ISSN XXXXX Composition of the wood oils of Calocedrus AJEONP 2013; 1 (1): 28-33 © 2013 AkiNik Publications macrolepis, Calocedrus rupestris and Received 16-7-2013 Cupressus tonkinensis (Cupressaceae) from Accepted: 20-8-2013 Vietnam Do N. Dai Do N. Dai, Tran D. Thang, Tran H. Thai, Bui V. Thanh, Isiaka A. Ogunwande Faculty of Biology, Vinh University, 182-Le Duan, Vihn City, Nghean ABSTRACT Province, Vietnam. E-mail: [email protected] In the present investigation we studied the essential oil contents and compositions of three individual plants from Cupressaceae family cultivated in Vietnam. The air-dried plants were hydrodistilled and Tran D. Thang the oils analysed by GC and GC-MS. The components were identified by MS libraries and their RIs. Faculty of Biology, Vinh University, The wood essential oil of Calocedrus rupestris Aver, H.T. Nguyen et L.K. Phan., afforded oil whose 182-Le Duan, Vihn City, Nghean major compounds were sesquiterpenes represented mainly by α-cedrol (31.1%) and thujopsene Province, Vietnam. E-mail: [email protected] (15.2%). In contrast, monoterpene compounds mainly α-terpineol (11.6%) and myrtenal (10.6%) occurred in Calocedrus macrolepis Kurz. The wood of Cupressus tonkinensis Silba afforded oil Tran H. Thai whose major compounds were also the monoterpenes namely sabinene (22.3%), -pinene (15.2%) Institute of Ecology and Biological and terpinen-4-ol (15.5%). The chemotaxonomic implication of the present results was also Resources, Vietnam Academy of discussed. Science and Technology, 18-Hoang Quoc Viet, Cau Giay, Hanoi, Keywords: Calocedrus macrolepis; Calocedrus rupestris; α-cedrol; Cupressus tonkinensis; Essential oil Vietnam. -

Spatial Distribution and Historical Dynamics of Threatened Conifers of the Dalat Plateau, Vietnam
SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM A thesis Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Master of Arts By TRANG THI THU TRAN Dr. C. Mark Cowell, Thesis Supervisor MAY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the thesis entitled SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM Presented by Trang Thi Thu Tran A candidate for the degree of Master of Arts of Geography And hereby certify that, in their opinion, it is worthy of acceptance. Professor C. Mark Cowell Professor Cuizhen (Susan) Wang Professor Mark Morgan ACKNOWLEDGEMENTS This research project would not have been possible without the support of many people. The author wishes to express gratitude to her supervisor, Prof. Dr. Mark Cowell who was abundantly helpful and offered invaluable assistance, support, and guidance. My heartfelt thanks also go to the members of supervisory committees, Assoc. Prof. Dr. Cuizhen (Susan) Wang and Prof. Mark Morgan without their knowledge and assistance this study would not have been successful. I also wish to thank the staff of the Vietnam Initiatives Group, particularly to Prof. Joseph Hobbs, Prof. Jerry Nelson, and Sang S. Kim for their encouragement and support through the duration of my studies. I also extend thanks to the Conservation Leadership Programme (aka BP Conservation Programme) and Rufford Small Grands for their financial support for the field work. Deepest gratitude is also due to Sub-Institute of Ecology Resources and Environmental Studies (SIERES) of the Institute of Tropical Biology (ITB) Vietnam, particularly to Prof. -

Monitoring the Emission of Volatile Organic Compounds from the Leaves of Calocedrus Macrolepis Var
J Wood Sci (2010) 56:140–147 © The Japan Wood Research Society 2009 DOI 10.1007/s10086-009-1071-z ORIGINAL ARTICLE Ying-Ju Chen · Sen-Sung Cheng · Shang-Tzen Chang Monitoring the emission of volatile organic compounds from the leaves of Calocedrus macrolepis var. formosana using solid-phase micro-extraction Received: June 10, 2009 / Accepted: August 17, 2009 / Published online: November 25, 2009 Abstract In this study, solid-phase micro-extraction through secondary metabolism in the process of growth and (SPME) fi bers coated with polydimethylsiloxane/divinyl- development. The terpenes derived from isoprenoids con- benzene (PDMS/DVB), coupled with gas chromatography/ stitute the largest class of secondary products, and they are mass spectrometry, were used to monitor the emission pat- also the most important precursors for phytoncides in forest terns of biogenic volatile organic compounds (BVOCs) materials. Phytoncides are volatile organic compounds from leaves of Calocedrus macrolepis var. formosana Florin. released by plants, and they resist and break up hazardous in situ. In both sunny and rainy weather, the circadian substances in the air. Scientists have confi rmed that phyt- profi le for BVOCs from C. macrolepis var. formosana oncides can reduce dust and bacteria in the air, and expo- leaves has three maximum emission cycles each day. This sure to essential oils from trees has also been reported to kind of emission pattern might result from the plant’s cir- lessen anxiety and depression, resulting in improved blood cadian clock, which determines the rhythm of terpenoid circulation and blood pressure reduction in humans and emission. Furthermore, emission results from the leaves animals.1 However, the chemical compositions of phyton- demonstrated that the circadian profi le of α-pinene observed cides emitted from various trees are very different and not was opposite to the profi les of limonene and myrcene, a yet clearly identifi ed. -

Morphology and Morphogenesis of the Seed Cones of the Cupressaceae - Part II Cupressoideae
1 2 Bull. CCP 4 (2): 51-78. (10.2015) A. Jagel & V.M. Dörken Morphology and morphogenesis of the seed cones of the Cupressaceae - part II Cupressoideae Summary The cone morphology of the Cupressoideae genera Calocedrus, Thuja, Thujopsis, Chamaecyparis, Fokienia, Platycladus, Microbiota, Tetraclinis, Cupressus and Juniperus are presented in young stages, at pollination time as well as at maturity. Typical cone diagrams were drawn for each genus. In contrast to the taxodiaceous Cupressaceae, in Cupressoideae outgrowths of the seed-scale do not exist; the seed scale is completely reduced to the ovules, inserted in the axil of the cone scale. The cone scale represents the bract scale and is not a bract- /seed scale complex as is often postulated. Especially within the strongly derived groups of the Cupressoideae an increased number of ovules and the appearance of more than one row of ovules occurs. The ovules in a row develop centripetally. Each row represents one of ascending accessory shoots. Within a cone the ovules develop from proximal to distal. Within the Cupressoideae a distinct tendency can be observed shifting the fertile zone in distal parts of the cone by reducing sterile elements. In some of the most derived taxa the ovules are no longer (only) inserted axillary, but (additionally) terminal at the end of the cone axis or they alternate to the terminal cone scales (Microbiota, Tetraclinis, Juniperus). Such non-axillary ovules could be regarded as derived from axillary ones (Microbiota) or they develop directly from the apical meristem and represent elements of a terminal short-shoot (Tetraclinis, Juniperus). -

Supporting Information
Supporting Information Mao et al. 10.1073/pnas.1114319109 SI Text BEAST Analyses. In addition to a BEAST analysis that used uniform Selection of Fossil Taxa and Their Phylogenetic Positions. The in- prior distributions for all calibrations (run 1; 144-taxon dataset, tegration of fossil calibrations is the most critical step in molecular calibrations as in Table S4), we performed eight additional dating (1, 2). We only used the fossil taxa with ovulate cones that analyses to explore factors affecting estimates of divergence could be assigned unambiguously to the extant groups (Table S4). time (Fig. S3). The exact phylogenetic position of fossils used to calibrate the First, to test the effect of calibration point P, which is close to molecular clocks was determined using the total-evidence analy- the root node and is the only functional hard maximum constraint ses (following refs. 3−5). Cordaixylon iowensis was not included in in BEAST runs using uniform priors, we carried out three runs the analyses because its assignment to the crown Acrogymno- with calibrations A through O (Table S4), and calibration P set to spermae already is supported by previous cladistic analyses (also [306.2, 351.7] (run 2), [306.2, 336.5] (run 3), and [306.2, 321.4] using the total-evidence approach) (6). Two data matrices were (run 4). The age estimates obtained in runs 2, 3, and 4 largely compiled. Matrix A comprised Ginkgo biloba, 12 living repre- overlapped with those from run 1 (Fig. S3). Second, we carried out two runs with different subsets of sentatives from each conifer family, and three fossils taxa related fi to Pinaceae and Araucariaceae (16 taxa in total; Fig. -

Gene Duplications and Genomic Conflict Underlie Major Pulses of Phenotypic 2 Evolution in Gymnosperms 3 4 Gregory W
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.13.435279; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Gene duplications and genomic conflict underlie major pulses of phenotypic 2 evolution in gymnosperms 3 4 Gregory W. Stull1,2,†, Xiao-Jian Qu3,†, Caroline Parins-Fukuchi4, Ying-Ying Yang1, Jun-Bo 5 Yang2, Zhi-Yun Yang2, Yi Hu5, Hong Ma5, Pamela S. Soltis6, Douglas E. Soltis6,7, De-Zhu Li1,2,*, 6 Stephen A. Smith8,*, Ting-Shuang Yi1,2,*. 7 8 1Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, 9 Kunming, Yunnan, China. 10 2CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of 11 Botany, Chinese Academy of Sciences, Kunming, China. 12 3Shandong Provincial Key Laboratory of Plant Stress Research, College of Life Sciences, 13 Shandong Normal University, Jinan, Shandong, China. 14 4Department of Geophysical Sciences, University of Chicago, Chicago, IL, USA. 15 5Department of Biology, Huck Institutes of the Life Sciences, Pennsylvania State University, 16 University Park, PA, USA. 17 6Florida Museum of Natural History, University of Florida, Gainesville, FL, USA. 18 7Department of Biology, University of Florida, Gainesville, FL, USA. 19 8Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, 20 MI, USA. 21 †Co-first author. 22 *Correspondence to: [email protected]; [email protected]; [email protected]. -
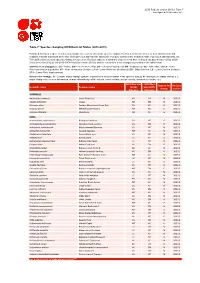
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

Number 4, Summer 1998 Director’S Letter Watering, Weeding, and Waiting by Bryce Lane Summer at the JC Raulston Arboretum Is a Great Time for Visiting
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 4, Summer 1998 Director’s Letter Watering, Weeding, and Waiting by Bryce Lane Summer at the JC Raulston Arboretum is a great time for visiting. However, be sure to bring plenty of water to drink. It has been a hot one so far, and that poses a few extra challenges for gardeners throughout the region. Our summer staff of Mitzi, Karen, Todd, Doug, and Sarah have been busy keeping the garden and collections in “tip top” shape. Much of their time has been involved in two typical summer time gardening Gala in the Garden Co-Chairs May McMillan Benson, left, and Peggy Fain share a well deserved smile during the very successful Gala. See page 18 for activities, watering and weeding. more gala news. photo by Linda Watson In between these activities they have been busy propagating a The giveaway is an incredible event insure the event was memorable. myriad of very interesting and where a “sea” of people select from Indeed it was a memorable Gala, exciting plants. That’s where the a “sea” of plants. It only takes about with brief gale force winds and waiting comes in! Many of these ten minutes for the plants to be torrential rains occurring right plants will be featured at the NC chosen and amazingly, no one is before dinner. That did not dampen Association of Nurserymen Trade injured or very disappointed! Be anyone’s spirits. As we all stood Show, which is now re-scheduled sure to mark your calendars. -

Induction of Compression Wood in Seedlings of Taiwan Incense Cedar (Calocedrus Macrolepis Var
Botanical Studies (2010) 51: 163-170. PHYSIOLOGY Induction of compression wood in seedlings of Taiwan incense cedar (Calocedrus macrolepis var. formosana) during the mid-season growth pause Ching-ChuTSAI1,Shiang-JiuunCHEN2,Ching-TeCHIEN3,*,andLing-LongKUO-HUANG1,2,* 1Institute of Ecology and Evolutionary Biology, National Taiwan University, 1 Roosevelt Rd., Sec. 4 Taipei 10617, Taiwan 2Department of Life Science, National Taiwan University, 1 Roosevelt Rd., Sec. 4 Taipei 10617, Taiwan 3Division of Silviculture, Taiwan Forestry Research Institute, 53 Nanhai Rd., Taipei 10066, Taiwan (ReceivedMarch10,2009;AcceptedNovember25,2009) ABSTRACT. Taiwanincensecedar, Calocedrus macrolepisvar.formosana,isanendemicvarietywith excellentwoodproperty.Howevertheannualradialgrowthisslowandwoodqualitywasfrequentlyabating bythepresenceofcompressionwood.Inthisstudy,amid-seasongrowthpausewasfoundinthebranches aswellastheseedlings.Theeffectsofgrowthpauseoncompressionwoodformationwereaccessed.The seedlingsofTaiwanincensecedarwereinducedtoformcompressionwoodbyindole-3-aceticacid(IAA) aswellashorizontallyleaninginthegrowthpause.Theresultsshowedthatalthoughthisgrowthpause decreasedthevascularcambialactivityinstandingcontrolseedlings,vascularcambiumofalltreatedseedlings reactedtotheinductionandexhibitedhighercambialactivityintheexpectedcompressionwoodformingside. TheslowannualradialgrowthofTaiwanincensecedarwasduetothetardygrowingnatureratherthanthe lengthofgrowingseason.Thisnaturemaycausetheslowpresentationrateofcompressionwoodobservedin differenttreatmentsinTaiwanincensecedar. -

4. CALOCEDRUS Kurz, J. Bot. 11: 196. 1873
Flora of China 4: 64–65. 1999. 4. CALOCEDRUS Kurz, J. Bot. 11: 196. 1873. 翠柏属 cui bai shu Trees evergreen, monoecious; branchlets arranged in a plane, spreading or ascending, flattened, prominently jointed. Leaves decussate, almost in whorls of 4, scalelike, base decurrent, dimorphic along branchlets: facial pairs flattened; lateral pairs boat-shaped, usually less than 4 mm, overlapping facial pairs, without conspicuous, white stomatal bands abaxially. Pollen cones terminal, solitary; microsporophylls 10–16, each with 2–5 pendulous pollen sacs. Seed cones terminal, solitary, oblong or ellipsoid-cylindric, dehiscent when mature in 1st year; cone scales 6, decussate, flat, only middle pair fertile, each fertile scale bearing 1 or 2 seeds; free bract apex a short mucro. Seeds with 2 subapical, unequal wings. Cotyledons 2. Two species: China, Laos, Myanmar, Thailand, Vietnam, Mexico, United States; one species in China. 1. Calocedrus macrolepis Kurz, J. Bot. 11: 196. 1873. 翠柏 cui bai Trees to 35 m tall; trunk to 1.5 m d.b.h.; bark grayish brown or reddish brown, smooth when young, fissured and exfoliating when old; crown pyramidal when young, broadly rounded when old; branches spreading and ascending. Leaves (1.5–)3–4(–8) mm. Pollen cones yellow, ovoid or oblong, 4–8 × 2–3 mm; microsporophylls each with (3 or)4(or 5) pollen sacs. Seed cones reddish brown when ripe, 10–20 × 4–6 mm; cone scales flattened, woody, fertile scales 2-seeded, basal pair small, ca. 3 mm, recurved, apical pair connate. Seeds subovoid or ellipsoid, slightly flattened, 5–6 mm. Pollination Mar–Apr, seed maturity Sep–Oct. -

Identification Key to the Cypress Family (Cupressaceae)1
Feddes Repertorium 116 (2005) 1–2, 96–146 DOI: 10.1002/fedr.200411062 Weinheim, Mai 2005 Ruhr-Universität Bochum, Lehrstuhl für Spezielle Botanik, Bochum C. SCHULZ; P. KNOPF & TH. STÜTZEL Identification key to the Cypress family (Cupressaceae)1 With 11 Figures Summary Zusammenfassung The identification of Cupressaceae taxa, except for Bestimmungsschlüssel für die Familie der Cup- some local and easily distinguishable taxa, is diffi- ressaceae cult even for specialists. One reason for this is the lack of a complete key including all Cupressaceae Die Bestimmung von Cupressaceae-Taxa ist mit taxa, another reason is that diagnoses and descrip- Ausnahme einiger lokaler und leicht bestimmbarer tions are spread over several hundred publications Taxa schwierig, selbst für Spezialisten. Ein Grund, which are sometimes difficult to access. Based on warum es noch keinen vollständigen Bestimmungs- morphological studies of about 3/4 of the species and schlüssel mit allen Cupressaceae-Taxa gibt ist, dass a careful compilation of the most important descrip- die Sippen-Beschreibungen sich auf mehrere hundert tions of Cupressaceae, a first identification key for Publikationen verteilen, welche teilweise schwierig the entire Cypress family (Cupressaceae) could be zu beschaffen sind. Etwa 3/4 der Cupressaceae-Ar- set up. The key comprises any of the 30 genera, 134 ten wurden morphologisch untersucht und die wich- species, 7 subspecies, 38 varieties, one form and thus tigsten Beschreibungen zusammengefasst, daraus all 180 taxa recognized by FARJON (2001). The key wurde dann der erste vollständige Bestimmungs- uses mainly features of adult leaves, female cones schlüssel für Cupressaceae erstellt. Der Bestim- and other characters which are all relatively easy to mungsschlüssel enthält 30 Gattungen, 134 Arten, be used.