Antitumor Agent Yatein from Calocedrus Formosana Florin Leaf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Composition of the Wood Oils of Calocedrus Macrolepis, Calocedrus
American Journal of Essential Oils and Natural Products 2013; 1 (1): 28-33 ISSN XXXXX Composition of the wood oils of Calocedrus AJEONP 2013; 1 (1): 28-33 © 2013 AkiNik Publications macrolepis, Calocedrus rupestris and Received 16-7-2013 Cupressus tonkinensis (Cupressaceae) from Accepted: 20-8-2013 Vietnam Do N. Dai Do N. Dai, Tran D. Thang, Tran H. Thai, Bui V. Thanh, Isiaka A. Ogunwande Faculty of Biology, Vinh University, 182-Le Duan, Vihn City, Nghean ABSTRACT Province, Vietnam. E-mail: [email protected] In the present investigation we studied the essential oil contents and compositions of three individual plants from Cupressaceae family cultivated in Vietnam. The air-dried plants were hydrodistilled and Tran D. Thang the oils analysed by GC and GC-MS. The components were identified by MS libraries and their RIs. Faculty of Biology, Vinh University, The wood essential oil of Calocedrus rupestris Aver, H.T. Nguyen et L.K. Phan., afforded oil whose 182-Le Duan, Vihn City, Nghean major compounds were sesquiterpenes represented mainly by α-cedrol (31.1%) and thujopsene Province, Vietnam. E-mail: [email protected] (15.2%). In contrast, monoterpene compounds mainly α-terpineol (11.6%) and myrtenal (10.6%) occurred in Calocedrus macrolepis Kurz. The wood of Cupressus tonkinensis Silba afforded oil Tran H. Thai whose major compounds were also the monoterpenes namely sabinene (22.3%), -pinene (15.2%) Institute of Ecology and Biological and terpinen-4-ol (15.5%). The chemotaxonomic implication of the present results was also Resources, Vietnam Academy of discussed. Science and Technology, 18-Hoang Quoc Viet, Cau Giay, Hanoi, Keywords: Calocedrus macrolepis; Calocedrus rupestris; α-cedrol; Cupressus tonkinensis; Essential oil Vietnam. -

Spatial Distribution and Historical Dynamics of Threatened Conifers of the Dalat Plateau, Vietnam
SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM A thesis Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Master of Arts By TRANG THI THU TRAN Dr. C. Mark Cowell, Thesis Supervisor MAY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the thesis entitled SPATIAL DISTRIBUTION AND HISTORICAL DYNAMICS OF THREATENED CONIFERS OF THE DALAT PLATEAU, VIETNAM Presented by Trang Thi Thu Tran A candidate for the degree of Master of Arts of Geography And hereby certify that, in their opinion, it is worthy of acceptance. Professor C. Mark Cowell Professor Cuizhen (Susan) Wang Professor Mark Morgan ACKNOWLEDGEMENTS This research project would not have been possible without the support of many people. The author wishes to express gratitude to her supervisor, Prof. Dr. Mark Cowell who was abundantly helpful and offered invaluable assistance, support, and guidance. My heartfelt thanks also go to the members of supervisory committees, Assoc. Prof. Dr. Cuizhen (Susan) Wang and Prof. Mark Morgan without their knowledge and assistance this study would not have been successful. I also wish to thank the staff of the Vietnam Initiatives Group, particularly to Prof. Joseph Hobbs, Prof. Jerry Nelson, and Sang S. Kim for their encouragement and support through the duration of my studies. I also extend thanks to the Conservation Leadership Programme (aka BP Conservation Programme) and Rufford Small Grands for their financial support for the field work. Deepest gratitude is also due to Sub-Institute of Ecology Resources and Environmental Studies (SIERES) of the Institute of Tropical Biology (ITB) Vietnam, particularly to Prof. -

Monitoring the Emission of Volatile Organic Compounds from the Leaves of Calocedrus Macrolepis Var
J Wood Sci (2010) 56:140–147 © The Japan Wood Research Society 2009 DOI 10.1007/s10086-009-1071-z ORIGINAL ARTICLE Ying-Ju Chen · Sen-Sung Cheng · Shang-Tzen Chang Monitoring the emission of volatile organic compounds from the leaves of Calocedrus macrolepis var. formosana using solid-phase micro-extraction Received: June 10, 2009 / Accepted: August 17, 2009 / Published online: November 25, 2009 Abstract In this study, solid-phase micro-extraction through secondary metabolism in the process of growth and (SPME) fi bers coated with polydimethylsiloxane/divinyl- development. The terpenes derived from isoprenoids con- benzene (PDMS/DVB), coupled with gas chromatography/ stitute the largest class of secondary products, and they are mass spectrometry, were used to monitor the emission pat- also the most important precursors for phytoncides in forest terns of biogenic volatile organic compounds (BVOCs) materials. Phytoncides are volatile organic compounds from leaves of Calocedrus macrolepis var. formosana Florin. released by plants, and they resist and break up hazardous in situ. In both sunny and rainy weather, the circadian substances in the air. Scientists have confi rmed that phyt- profi le for BVOCs from C. macrolepis var. formosana oncides can reduce dust and bacteria in the air, and expo- leaves has three maximum emission cycles each day. This sure to essential oils from trees has also been reported to kind of emission pattern might result from the plant’s cir- lessen anxiety and depression, resulting in improved blood cadian clock, which determines the rhythm of terpenoid circulation and blood pressure reduction in humans and emission. Furthermore, emission results from the leaves animals.1 However, the chemical compositions of phyton- demonstrated that the circadian profi le of α-pinene observed cides emitted from various trees are very different and not was opposite to the profi les of limonene and myrcene, a yet clearly identifi ed. -

Maquette En Jn:Gestion Durable.Qxd.Qxd
Appendices 119 A PPENDIX 1 List and origins of quantitative SFM indicators in 2005 Topic N° Full indicator Origin C1: Maintenance and appropriate enhancement of forest resources and their contribution to global carbon cycles Forest area 1.1 Area of forest and other wooded land, classified by forest type and by availability for MCPFE Vienna wood supply 1.1.1 Forest area gains and losses ISFM 2000 1.1.2 Forest area by biogeographical area and elevation class ISFM 2000 1.1.3 Forest area by IFN forest structure ISFM 2000 1.1.4 Forest area by main tree species ISFM 2000 Growing stock 1.2 Growing stock on forest and other wooded land, classified by forest type and by avai- MCPFE Vienna lability for wood supply 1.2.1 Growing stock by IFN forest structure ISFM 2000 1.2.2 Growing stock by tree species ISFM 2000 Age structure and/or 1.3 Age structure and/or diameter distribution of forest and other wooded land, classified MCPFE Vienna diameter distribution by forest type and by availability for wood supply Carbon stock 1.4 Carbon stock of woody biomass and of soils on forest and other wooded land MCPFE Vienna 1.4.1 Annual carbon emission levels ISFM 2000 C2: Maintenance of forest ecosystem health and vitality Deposition of air pollu- 2.1 Deposition of air pollutants on forest and other wooded land, classified by N, S and MCPFE Vienna tants base cations 2.1.1 Atmospheric pollutant emission patterns ISFM 2000 Soil condition 2.2 Chemical soil properties (pH, CEC, C/N, organic C, base saturation) on forest and MCPFE Vienna other wooded land related -

Morphology and Morphogenesis of the Seed Cones of the Cupressaceae - Part II Cupressoideae
1 2 Bull. CCP 4 (2): 51-78. (10.2015) A. Jagel & V.M. Dörken Morphology and morphogenesis of the seed cones of the Cupressaceae - part II Cupressoideae Summary The cone morphology of the Cupressoideae genera Calocedrus, Thuja, Thujopsis, Chamaecyparis, Fokienia, Platycladus, Microbiota, Tetraclinis, Cupressus and Juniperus are presented in young stages, at pollination time as well as at maturity. Typical cone diagrams were drawn for each genus. In contrast to the taxodiaceous Cupressaceae, in Cupressoideae outgrowths of the seed-scale do not exist; the seed scale is completely reduced to the ovules, inserted in the axil of the cone scale. The cone scale represents the bract scale and is not a bract- /seed scale complex as is often postulated. Especially within the strongly derived groups of the Cupressoideae an increased number of ovules and the appearance of more than one row of ovules occurs. The ovules in a row develop centripetally. Each row represents one of ascending accessory shoots. Within a cone the ovules develop from proximal to distal. Within the Cupressoideae a distinct tendency can be observed shifting the fertile zone in distal parts of the cone by reducing sterile elements. In some of the most derived taxa the ovules are no longer (only) inserted axillary, but (additionally) terminal at the end of the cone axis or they alternate to the terminal cone scales (Microbiota, Tetraclinis, Juniperus). Such non-axillary ovules could be regarded as derived from axillary ones (Microbiota) or they develop directly from the apical meristem and represent elements of a terminal short-shoot (Tetraclinis, Juniperus). -

Supporting Information
Supporting Information Mao et al. 10.1073/pnas.1114319109 SI Text BEAST Analyses. In addition to a BEAST analysis that used uniform Selection of Fossil Taxa and Their Phylogenetic Positions. The in- prior distributions for all calibrations (run 1; 144-taxon dataset, tegration of fossil calibrations is the most critical step in molecular calibrations as in Table S4), we performed eight additional dating (1, 2). We only used the fossil taxa with ovulate cones that analyses to explore factors affecting estimates of divergence could be assigned unambiguously to the extant groups (Table S4). time (Fig. S3). The exact phylogenetic position of fossils used to calibrate the First, to test the effect of calibration point P, which is close to molecular clocks was determined using the total-evidence analy- the root node and is the only functional hard maximum constraint ses (following refs. 3−5). Cordaixylon iowensis was not included in in BEAST runs using uniform priors, we carried out three runs the analyses because its assignment to the crown Acrogymno- with calibrations A through O (Table S4), and calibration P set to spermae already is supported by previous cladistic analyses (also [306.2, 351.7] (run 2), [306.2, 336.5] (run 3), and [306.2, 321.4] using the total-evidence approach) (6). Two data matrices were (run 4). The age estimates obtained in runs 2, 3, and 4 largely compiled. Matrix A comprised Ginkgo biloba, 12 living repre- overlapped with those from run 1 (Fig. S3). Second, we carried out two runs with different subsets of sentatives from each conifer family, and three fossils taxa related fi to Pinaceae and Araucariaceae (16 taxa in total; Fig. -

Leaf Extracts of Calocedrus Formosana (Florin) Induce G2/M Cell Cycle Arrest and Apoptosis in Human Bladder Cancer Cells
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2011, Article ID 380923, 10 pages doi:10.1155/2011/380923 Research Article Leaf Extracts of Calocedrus formosana (Florin) Induce G2/M Cell Cycle Arrest and Apoptosis in Human Bladder Cancer Cells Sheau-Yun Yuan,1, 2 Chi-Chen Lin,3, 4 Shih-Lan Hsu,3 Ya-Wen Cheng,1 Jyh-Horng Wu,5 Chen-Li Cheng,2 and Chi-Rei Yang2 1 Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan 2 Division of Urology, Department of Surgery, Taichung Veterans General Hospital, Taichung 40705, Taiwan 3 Department of Education and Research, Taichung Veterans General Hospital, Taichung 40705, Taiwan 4 Institute of Biomedical Science, National Chung-Hsing University, Taichung 40227, Taiwan 5 Department of Forestry, National Chung-Hsing University, Taichung 40227, Taiwan Correspondence should be addressed to Chen-Li Cheng, [email protected] and Chi-Rei Yang, [email protected] Received 14 December 2010; Revised 14 March 2011; Accepted 24 March 2011 Copyright © 2011 Sheau-Yun Yuan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calocedrus formosana (Florin) bark acetone/ethylacetate extracts are known to exert an antitumor effect on some human cancer cell lines, but the mechanism is yet to be defined. The aim of this study was to determine the effects of Florin leaf methanol extracts on the growth and apoptosis of human bladder cancer cell lines. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay showed that the growth of these bladder cancer cells was potently inhibited by the Florin leaf extracts. -

Comparison of Soil Bacterial Communities in a Natural Hardwood
Lin et al. Botanical Studies 2014, 55:50 http://www.as-botanicalstudies.com/content/55/1/50 RESEARCH Open Access Comparison of soil bacterial communities in a natural hardwood forest and coniferous plantations in perhumid subtropical low mountains Yu-Te Lin1, Hsueh-Wen Hu1, William B Whitman2, David C Coleman3 and Chih-Yu Chiu1* Abstract Background: The bacterial community of forest soils is influenced by environmental disturbance and/or meteorological temperature and precipitation. In this study, we investigated three bacterial communities in soils of a natural hardwood forest and two plantations of conifer, Calocedrus formosana and Cryptomeria japonica,ina perhumid, low mountain area. By comparison with our previous studies with similar temperature and/or precipitation, we aimed to elucidate how disturbance influences the bacterial community in forest soils and whether bacterial communities in similar forest types differ under different climate conditions. Results: Analysis of 16S ribosomal RNA gene clone libraries revealed that Acidobacteria and Proteobacteria were the most abundant phyla in the three forest soil communities, with similar relative abundance of various bacterial groups. However, UniFrac analysis based on phylogenetic information revealed differences of bacterial communities between natural hardwood forest and coniferous plantation soils. The diversities of bacterial communities of the replanted Calocedrus and Cryptomeria forests were higher than that in natural hardwood forest. The bacterial diversity of these three forest soil were all higher than those in the same forest types at other locations with less precipitation or with lower temperature. In addition, the distribution of some of the most abundant operational taxonomic units in the three communities differed from other forest soils, including those related to Acidobacteria, α-, β- and γ-Proteobacteria. -

ANTITERMITIC and ANTIFUNGAL ACTIVITIES of ESSENTIAL OIL of Calocedrus Formosana LEAF and ITS COMPOSITION
Journal of Chemical Ecology, Vol. 30, No. 10, October 2004 (C 2004) ANTITERMITIC AND ANTIFUNGAL ACTIVITIES OF ESSENTIAL OIL OF Calocedrus formosana LEAF AND ITS COMPOSITION SEN-SUNG CHENG, CHI-LIN WU, HUI-TING CHANG, YU-TING KAO, and SHANG-TZEN CHANG∗ School of Forestry and Resource Conservation National Taiwan University, No. 1, Section 4 Roosevelt Road, Taipei 106, Taiwan (Received November 4, 2003; accepted June 16, 2004) Abstract—Calocedrus formosana Florin (Cupressaceae) is an endemic tree species in Taiwan; its timber is recognized for natural decay resistance. To ex- amine the antitermitic and antifungal activities of leaf essential oil and its main constituents, C. formosana leaves were extracted and the essential oils ana- lyzed by GC-MS. Bioactivity tests against the termite Coptotermes formosanus demonstrate that the LC50 value of leaf essential oil is 27.6 mg/g. Furthermore, exposure to T-muurolol caused 100% mortality at a dosage of 5 mg/g after 14 d. Leaf oil constituents displayed activity against four fungi, Lenzites betulina, Pycnoporus coccineus, Trametes versicolor,andLaetiporus sulphureus.Two compounds, α-cadinol and T-muurolol, exhibited the strongest antifungal ac- tivity. The LC50 values of α-cadinol against L. sulphureus, L. betulina,andT. versicolor are 9.9, 28.6, and 30.4 µg/ml, respectively. Key Words—Calocedrus formosana, leaf, essential oil, GC-MS, Coptotermes formosanus, antitermitic activity, antifungal activity, α-cadinol, T-muurolol. INTRODUCTION Wood, a naturally occurring polymer composite, is mainly composed of cellulose, hemicelluloses, lignin, and extractives. Due to its biological nature, unprotected wood is susceptible to discoloration and biological deterioration, which reduce its mechanical and physical properties (Chang et al., 2002). -

Gene Duplications and Genomic Conflict Underlie Major Pulses of Phenotypic 2 Evolution in Gymnosperms 3 4 Gregory W
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.13.435279; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Gene duplications and genomic conflict underlie major pulses of phenotypic 2 evolution in gymnosperms 3 4 Gregory W. Stull1,2,†, Xiao-Jian Qu3,†, Caroline Parins-Fukuchi4, Ying-Ying Yang1, Jun-Bo 5 Yang2, Zhi-Yun Yang2, Yi Hu5, Hong Ma5, Pamela S. Soltis6, Douglas E. Soltis6,7, De-Zhu Li1,2,*, 6 Stephen A. Smith8,*, Ting-Shuang Yi1,2,*. 7 8 1Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, 9 Kunming, Yunnan, China. 10 2CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of 11 Botany, Chinese Academy of Sciences, Kunming, China. 12 3Shandong Provincial Key Laboratory of Plant Stress Research, College of Life Sciences, 13 Shandong Normal University, Jinan, Shandong, China. 14 4Department of Geophysical Sciences, University of Chicago, Chicago, IL, USA. 15 5Department of Biology, Huck Institutes of the Life Sciences, Pennsylvania State University, 16 University Park, PA, USA. 17 6Florida Museum of Natural History, University of Florida, Gainesville, FL, USA. 18 7Department of Biology, University of Florida, Gainesville, FL, USA. 19 8Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, 20 MI, USA. 21 †Co-first author. 22 *Correspondence to: [email protected]; [email protected]; [email protected]. -
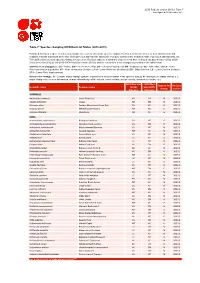
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change.