A Combined Morphological and Molecular Evolutionary Analysis of Karst-Environment Adaptation for the Genus Urophysa (Ranunculaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Chloroplast Genome Sequence of Boswellia Sacra, a Resin-Producing Plant in Oman
RESEARCH ARTICLE The First Chloroplast Genome Sequence of Boswellia sacra, a Resin-Producing Plant in Oman Abdul Latif Khan1, Ahmed Al-Harrasi1*, Sajjad Asaf2, Chang Eon Park2, Gun-Seok Park2, Abdur Rahim Khan2, In-Jung Lee2, Ahmed Al-Rawahi1, Jae-Ho Shin2* 1 UoN Chair of Oman's Medicinal Plants & Marine Natural Products, University of Nizwa, Nizwa, Oman, 2 School of Applied Biosciences, Kyungpook National University, Daegu, Republic of Korea a1111111111 * [email protected] (AAH); [email protected] (JHS) a1111111111 a1111111111 a1111111111 Abstract a1111111111 Boswellia sacra (Burseraceae), a keystone endemic species, is famous for the production of fragrant oleo-gum resin. However, the genetic make-up especially the genomic informa- tion about chloroplast is still unknown. Here, we described for the first time the chloroplast OPEN ACCESS (cp) genome of B. sacra. The complete cp sequence revealed a circular genome of 160,543 Citation: Khan AL, Al-Harrasi A, Asaf S, Park CE, bp size with 37.61% GC content. The cp genome is a typical quadripartite chloroplast struc- Park G-S, Khan AR, et al. (2017) The First ture with inverted repeats (IRs 26,763 bp) separated by small single copy (SSC; 18,962 bp) Chloroplast Genome Sequence of Boswellia sacra, and large single copy (LSC; 88,055 bp) regions. De novo assembly and annotation showed a Resin-Producing Plant in Oman. PLoS ONE 12 the presence of 114 unique genes with 83 protein-coding regions. The phylogenetic analysis (1): e0169794. doi:10.1371/journal.pone.0169794 revealed that the B. sacra cp genome is closely related to the cp genome of Azadirachta Editor: Xiu-Qing Li, Agriculture and Agri-Food indica and Citrus sinensis, while most of the syntenic differences were found in the non-cod- Canada, CANADA ing regions. -

Phylogenetic Position and Generic Limits of Arabidopsis (Brassicaceae)
PHYLOGENETIC POSITION Steve L. O'Kane, Jr.2 and Ihsan A. 3 AND GENERIC LIMITS OF Al-Shehbaz ARABIDOPSIS (BRASSICACEAE) BASED ON SEQUENCES OF NUCLEAR RIBOSOMAL DNA1 ABSTRACT The primary goals of this study were to assess the generic limits and monophyly of Arabidopsis and to investigate its relationships to related taxa in the family Brassicaceae. Sequences of the internal transcribed spacer region (ITS-1 and ITS-2) of nuclear ribosomal DNA, including 5.8S rDNA, were used in maximum parsimony analyses to construct phylogenetic trees. An attempt was made to include all species currently or recently included in Arabidopsis, as well as species suggested to be close relatives. Our ®ndings show that Arabidopsis, as traditionally recognized, is polyphyletic. The genus, as recircumscribed based on our results, (1) now includes species previously placed in Cardaminopsis and Hylandra as well as three species of Arabis and (2) excludes species now placed in Crucihimalaya, Beringia, Olimar- abidopsis, Pseudoarabidopsis, and Ianhedgea. Key words: Arabidopsis, Arabis, Beringia, Brassicaceae, Crucihimalaya, ITS phylogeny, Olimarabidopsis, Pseudoar- abidopsis. Arabidopsis thaliana (L.) Heynh. was ®rst rec- netic studies and has played a major role in un- ommended as a model plant for experimental ge- derstanding the various biological processes in netics over a half century ago (Laibach, 1943). In higher plants (see references in Somerville & Mey- recent years, many biologists worldwide have fo- erowitz, 2002). The intraspeci®c phylogeny of A. cused their research on this plant. As indicated by thaliana has been examined by Vander Zwan et al. Patrusky (1991), the widespread acceptance of A. (2000). Despite the acceptance of A. -

PYK10 Myrosinase Reveals a Functional Coordination Between Endoplasmic Reticulum Bodies and Glucosinolates in Arabidopsis Thaliana
The Plant Journal (2017) 89, 204–220 doi: 10.1111/tpj.13377 PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana Ryohei T. Nakano1,2,3, Mariola Pislewska-Bednarek 4, Kenji Yamada5,†, Patrick P. Edger6,‡, Mado Miyahara3,§, Maki Kondo5, Christoph Bottcher€ 7,¶, Masashi Mori8, Mikio Nishimura5, Paul Schulze-Lefert1,2,*, Ikuko Hara-Nishimura3,*,#,k and Paweł Bednarek4,*,# 1Department of Plant Microbe Interactions, Max Planck Institute for Plant Breeding Research, Carl-von-Linne-Weg 10, D-50829 Koln,€ Germany, 2Cluster of Excellence on Plant Sciences (CEPLAS), Max Planck Institute for Plant Breeding Research, Carl-von-Linne-Weg 10, D-50829 Koln,€ Germany, 3Department of Botany, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, 4Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, 61-704 Poznan, Poland, 5Department of Cell Biology, National Institute of Basic Biology, Okazaki 444-8585, Japan, 6Department of Plant and Microbial Biology, University of California, Berkeley, CA 94720, USA, 7Department of Stress and Developmental Biology, Leibniz Institute of Plant Biochemistry, D-06120 Halle (Saale), Germany, and 8Ishikawa Prefectural University, Nonoichi, Ishikawa 834-1213, Japan Received 29 March 2016; revised 30 August 2016; accepted 5 September 2016; published online 19 December 2016. *For correspondence (e-mails [email protected]; [email protected]; [email protected]). #These authors contributed equally to this work. †Present address: Malopolska Centre of Biotechnology, Jagiellonian University, 30-387 Krakow, Poland. ‡Present address: Department of Horticulture, Michigan State University, East Lansing, MI, USA. §Present address: Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan. -

Chromosome‐Level Reference Genome of the Soursop (Annona Muricata
Received: 26 May 2020 | Revised: 5 February 2021 | Accepted: 8 February 2021 DOI: 10.1111/1755-0998.13353 RESOURCE ARTICLE Chromosome- level reference genome of the soursop (Annona muricata): A new resource for Magnoliid research and tropical pomology Joeri S. Strijk1,2,3 | Damien D. Hinsinger2,4 | Mareike M. Roeder5,6 | Lars W. Chatrou7 | Thomas L.P. Couvreur8 | Roy H.J. Erkens9 | Hervé Sauquet10 | Michael D. Pirie11 | Daniel C. Thomas12 | Kunfang Cao13 1Institute for Biodiversity and Environmental Research, Universiti Brunei Darussalam, Jalan Tungku Link, Brunei Darussalam 2Alliance for Conservation Tree Genomics, Pha Tad Ke Botanical Garden, Luang Prabang, Laos 3Guangxi Key Laboratory of Forest Ecology and Conservation, Biodiversity Genomics Team, Nanning, Guangxi, China 4Génomique Métabolique, Genoscope, Institut de Biologie François Jacob, Commissariat à l′Énergie Atomique (CEA), CNRS, Université Évry, Université Paris- Saclay, Évry, France 5Community Ecology and Conservation Group, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan, China 6Aueninstitut, Institute for Geography and Geoecology, Karlsruhe Institute of Technology, Rastatt, Germany 7Systematic and Evolutionary Botany Laboratory, Ghent University, Ghent, Belgium 8IRD, DIADE, Univ Montpellier, Montpellier, France 9Maastricht Science Programme, Maastricht University, Maastricht, The Netherlands 10National Herbarium of New South Wales (NSW), Royal Botanic Gardens and Domain Trust, Sydney, NSW, Australia 11Department of Natural History, -

Reproductive Morphology of Sargentodoxa Cuneata (Lardizabalaceae) and Its Systematic Implications
Reproductive morphology of Sargentodoxa cuneata (Lardizabalaceae) and its systematic implications. By: Hua-Feng Wang, Bruce K. Kirchoff and Zhi-Xin Zhu Wang, H.-F., Kirchoff, B. K., Qin, H.-N., Zhu, Z.-X. 2009. Reproductive morphology of Sargentodoxa cuneata (Lardizabalaceae) and its systematic implications. Plant Systematics and Evolution 280: 207–217. Made available courtesy of Springer-Verlag. The original publication is available at http://link.springer.com/article/10.1007%2Fs00606-009-0179-3. ***Reprinted with permission. No further reproduction is authorized without written permission from Springer-Verlag. This version of the document is not the version of record. Figures and/or pictures may be missing from this format of the document. *** Abstract: The reproductive morphology of Sargentodoxa cuneata (Oliv) Rehd. et Wils. is investigated through field, herbarium, and laboratory observations. Sargentodoxa may be either dioecious or monoecious. The functionally unisexual flowers are morphologically bisexual, at least developmentally. The anther is tetrasporangiate, and its wall, of which the development follows the basic type, is composed of an epidermis, endothecium, two middle layers, and a tapetum. The tapetum is of the glandular type. Microspore cytokinesis is simultaneous, and the microspore tetrads are tetrahedral. Pollen grains are two-celled when shed. The mature ovule is crassinucellate and bitegmic, and the micropyle is formed only by the inner integument. Megasporocytes undergo meiosis resulting in the formation of four megaspores in a linear tetrad. The functional megaspore develops into an eight-nucleate embryo sac after three rounds of mitosis. The mature embryo sac consists of an egg apparatus (an egg and two synergids), a central cell, and three antipodal cells. -

Wood and Bark Anatomy of Ranunculaceae (Including Hydrastis) and Glaucidiaceae Sherwin Carlquist Santa Barbara Botanic Garden
Aliso: A Journal of Systematic and Evolutionary Botany Volume 14 | Issue 2 Article 2 1995 Wood and Bark Anatomy of Ranunculaceae (Including Hydrastis) and Glaucidiaceae Sherwin Carlquist Santa Barbara Botanic Garden Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Carlquist, Sherwin (1995) "Wood and Bark Anatomy of Ranunculaceae (Including Hydrastis) and Glaucidiaceae," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 14: Iss. 2, Article 2. Available at: http://scholarship.claremont.edu/aliso/vol14/iss2/2 Aliso, 14(2), pp. 65-84 © 1995, by The Rancho Santa Ana Botanic Garden, Claremont, CA 91711-3157 WOOD AND BARK ANATOMY OF RANUNCULACEAE (INCLUDING HYDRASTIS) AND GLAUCIDIACEAE SHERWIN CARLQUIST Santa Barbara Botanic Garden 1212 Mission Canyon Road Santa Barbara, California 931051 ABSTRACT Wood anatomy of 14 species of Clematis and one species each of Delphinium, Helleborus, Thal ictrum, and Xanthorhiza (Ranunculaceae) is compared to that of Glaucidium palma tum (Glaucidiaceae) and Hydrastis canadensis (Ranunculaceae, or Hydrastidaceae of some authors). Clematis wood has features typical of wood of vines and lianas: wide (earlywood) vessels, abundant axial parenchyma (earlywood, some species), high vessel density, low proportion of fibrous tissue in wood, wide rays composed of thin-walled cells, and abrupt origin of multiseriate rays. Superimposed on these features are expressions indicative of xeromorphy in the species of cold or dry areas: numerous narrow late wood vessels, presence of vasicentric tracheids, shorter vessel elements, and strongly marked growth rings. Wood of Xanthorhiza is like that of a (small) shrub. Wood of Delphinium, Helleborus, and Thalictrum is characteristic of herbs that become woodier: limited amounts of secondary xylem, par enchymatization of wood, partial conversion of ray areas to libriform fibers (partial raylessness). -

POPOVICH, Encoding a C2H2 Zinc-Finger Transcription Factor, Plays
POPOVICH, encoding a C2H2 zinc-finger transcription factor, plays a central role in the development of a key innovation, floral nectar spurs, in Aquilegia Evangeline S. Ballerinia,1,2 , Ya Minb , Molly B. Edwardsb, Elena M. Kramerb , and Scott A. Hodgesa,1 aEcology, Evolution and Marine Biology Department, University of California, Santa Barbara, CA 93106; and bDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02318 Edited by Dominique C. Bergmann, Stanford University, Stanford, CA, and approved July 30, 2020 (received for review April 11, 2020) The evolution of novel features, such as eyes or wings, that allow traits, such as the powered flight of insects, birds, and bats, or organisms to exploit their environment in new ways can lead the pharyngeal jaws of cichlid fish, is recognized as contribut- to increased diversification rates. Therefore, understanding the ing to lineage diversification (13–15), discovering the genetic genetic and developmental mechanisms involved in the origin and developmental mechanisms that led to their evolution is of these key innovations has long been of interest to evolution- often difficult, in part, because many of these traits involve com- ary biologists. In flowering plants, floral nectar spurs are a prime plex developmental mechanisms that arose deep in evolutionary example of a key innovation, with the independent evolution of history. Given that the Aquilegia nectar spur evolved relatively spurs associated with increased diversification rates in multiple recently and is formed by modifications to a single floral organ, angiosperm lineages due to their ability to promote reproductive it provides a unique opportunity to begin to dissect the develop- isolation via pollinator specialization. -

Ecological Aspects of the Cretaceous Flowering Plant Radiation1
Aww. Akv. EarfA f/o««f. Sci. 799& 26. J7V-/J/ ECOLOGICAL ASPECTS OF THE CRETACEOUS FLOWERING PLANT RADIATION1 Scott L. Wing and Lisa D. Boucher Department of Paleobiology, NHB121, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560; e-mail: [email protected], boucherl @ nmnh. si.edu KEY WORDS: angiosperms, Cretaceous, paleoecology, diversification, evolution ABSTRACT The first flowering plant fossils occur as rare, undiverse pollen grains in the Early Cretaceous (Valanginian-Hauterivian). Angiosperms diversified slowly during the Barremian-Aptian but rapidly during the Albian-Cenomanian. By the end of the Cretaceous, at least half of the living angiosperm orders were present, and angiosperms were greater than 70% of terrestrial plant species globally. The rapid diversification of the group, and its dominance in modern vegetation, has led to the idea that the Cretaceous radiation of angiosperms also represents their rise to vegetational dominance. Paleoecological data cast a different light on the Cretaceous radiation of an- giosperms. Analyses of sedimentary environments indicate that angiosperms not only originated in unstable habitats but remained centered there through most of the Cretaceous. Morphology of leaves, seeds, and wood is consistent with the status of most Cretaceous angiosperms as herbs to small trees with early successional strategy. The diversification of flowering plants in the Cretaceous represents the evolution of a highly speciose clade of weeds but not necessarily a major change in global vegetation. INTRODUCTION Flowering plants, or angiosperms, are presently the most dominant group of terrestrial autotrophs. They span an array of habits including very large trees, 1 The US Government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper. -

A Chromosome-Scale Reference Genome of Aquilegia Oxysepala Var
Xie et al. Horticulture Research (2020) 7:113 Horticulture Research https://doi.org/10.1038/s41438-020-0328-y www.nature.com/hortres ARTICLE Open Access A chromosome-scale reference genome of Aquilegia oxysepala var. kansuensis Jinghe Xie1,2, Haifeng Zhao1,2, Kunpeng Li1,2, Rui Zhang1, Yongchao Jiang1,MeimeiWang1,2, Xuelian Guo1,BenYu1,2, Hongzhi Kong 1,2, Yuannian Jiao 1,2 and Guixia Xu 1,2 Abstract The genus Aquilegia (Ranunculaceae) has been cultivated as ornamental and medicinal plants for centuries. With petal spurs of strikingly diverse size and shape, Aquilegia has also been recognized as an excellent system for evolutionary studies. Pollinator‐mediated selection for longer spurs is believed to have shaped the evolution of this genus, especially the North American taxa. Recently, however, an opposite evolutionary trend was reported in an Asian lineage, where multiple origins of mini- or even nonspurred morphs have occurred. Interesting as it is, the lack of genomic resources has limited our ability to decipher the molecular and evolutionary mechanisms underlying spur reduction in this special lineage. Using long-read sequencing (PacBio Sequel), in combination with optical maps (BioNano DLS) and Hi–C, we assembled a high-quality reference genome of A. oxysepala var. kansuensis, a sister species to the nonspurred taxon. The final assembly is approximately 293.2 Mb, 94.6% (277.4 Mb) of which has been anchored to 7 pseudochromosomes. A total of 25,571 protein-coding genes were predicted, with 97.2% being functionally annotated. When comparing this genome with that of A. coerulea, we detected a large rearrangement between Chr1 and Chr4, which might have caused the Chr4 of A. -

China: a Rich Flora Needed of Urgent Conservationprovided by Digital.CSIC
Orsis 19, 2004 49-89 View metadata, citation and similar papers at core.ac.uk brought to you by CORE China: a rich flora needed of urgent conservationprovided by Digital.CSIC López-Pujol, Jordi GReB, Laboratori de Botànica, Facultat de Farmàcia, Universitat de Barcelona, Avda. Joan XXIII s/n, E-08028, Barcelona, Catalonia, Spain. Author for correspondence (E-mail: [email protected]) Zhao, A-Man Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, The People’s Republic of China. Manuscript received in april 2004 Abstract China is one of the richest countries in plant biodiversity in the world. Besides to a rich flora, which contains about 33 000 vascular plants (being 30 000 of these angiosperms, 250 gymnosperms, and 2 600 pteridophytes), there is a extraordinary ecosystem diversity. In addition, China also contains a large pool of both wild and cultivated germplasm; one of the eight original centers of crop plants in the world was located there. China is also con- sidered one of the main centers of origin and diversification for seed plants on Earth, and it is specially profuse in phylogenetically primitive taxa and/or paleoendemics due to the glaciation refuge role played by this area in the Quaternary. The collision of Indian sub- continent enriched significantly the Chinese flora and produced the formation of many neoen- demisms. However, the distribution of the flora is uneven, and some local floristic hotspots can be found across China, such as Yunnan, Sichuan and Taiwan. Unfortunately, threats to this biodiversity are huge and have increased substantially in the last 50 years. -
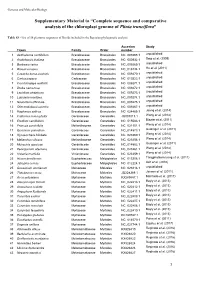
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

POPOVICH, Encoding a C2H2 Zinc-Finger Transcription Factor, Plays a Central Role in the Development of a Key Innovation, Floral
POPOVICH, encoding a C2H2 zinc-finger transcription factor, plays a central role in the development of a key innovation, floral nectar spurs, in Aquilegia Evangeline S. Ballerinia,1,2 , Ya Minb , Molly B. Edwardsb, Elena M. Kramerb , and Scott A. Hodgesa,1 aEcology, Evolution and Marine Biology Department, University of California, Santa Barbara, CA 93106; and bDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02318 Edited by Dominique C. Bergmann, Stanford University, Stanford, CA, and approved July 30, 2020 (received for review April 11, 2020) The evolution of novel features, such as eyes or wings, that allow traits, such as the powered flight of insects, birds, and bats, or organisms to exploit their environment in new ways can lead the pharyngeal jaws of cichlid fish, is recognized as contribut- to increased diversification rates. Therefore, understanding the ing to lineage diversification (13–15), discovering the genetic genetic and developmental mechanisms involved in the origin and developmental mechanisms that led to their evolution is of these key innovations has long been of interest to evolution- often difficult, in part, because many of these traits involve com- ary biologists. In flowering plants, floral nectar spurs are a prime plex developmental mechanisms that arose deep in evolutionary example of a key innovation, with the independent evolution of history. Given that the Aquilegia nectar spur evolved relatively spurs associated with increased diversification rates in multiple recently and is formed by modifications to a single floral organ, angiosperm lineages due to their ability to promote reproductive it provides a unique opportunity to begin to dissect the develop- isolation via pollinator specialization.