Enterohepatic Helicobacter Other Than Helicobacter Pylori
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kongre Kitabı
Book of Abstracts International VETistanbul Group Congress 2014 28-30 April, 2014 Istanbul, Turkey Book of Abstracts www.vetistanbul2014.org International VETistanbul Group Congress 2014 28-30 April 2014 International VETistanbul Group Congress 2014 28-30 April, 2014 Istanbul, Turkey Organizing Committee Prof. Dr. Halil GÜNEŞ, Chair Prof. Dr. Bülent EKİZ Prof. Dr. Ali AYDIN Assoc. Prof. Dr. Serkan İKİZ Assoc. Prof. Dr. Hasret DEMİRCAN YARDİBİ Assoc. Prof. Dr. Gülsün PAZVANT Scientific Committee* Prof. Dr. Kemal AK, Turkey Prof. Dr. Anatoliy ALEXANDROVICH STEKOLNIKOV, Russia Prof. Dr. Bogdan AMINKOV, Bulgaria Prof. Dr. Geno ATASANOV ANGELOV, Bulgaria Prof. Dr. Hajrudin BESIROVIC, Bosnia and Herzegovina Prof. Dr. Nihad FEJZIC, Bosnia and Herzegovina Assoc. Prof. Dr. Plamen GEORGIEV, Bulgaria Prof. Dr. Zehra HAJRULAI MUSLIU, Macedonia Assoc. Prof. Dr. Afrim HAMIDI, Kosovo Prof. Dr. Telman ISKENDEROV, Azerbaijan Prof. Dr. Larisa KARPENKO, Russia Prof. Dr. Ismail KIRSAN, Turkey Prof. Dr. Mihni LYUTSKANOV, Bulgaria Assoc. Prof. Dr. Avni ROBAJ, Kosovo Prof. Dr. Velimir STOJKOVSKI, Macedonia Prof. Dr. Semsir VELIYEV, Azerbaijan *Alphabetically listed by the according to the family name Scientific Secreteria Prof. Dr. Bülent EKİZ, Turkey Dr. Karlo MURATOĞLU, Turkey International VETistanbul Group Congress 2014, 28-30 April, Istanbul, Turkey IV International VETistanbul Group Congress 2014 28-30 April 2014 Dear Respectable Colleagues and Guests, First of all, I greet you all with my heart. Also, I would like to thank you for taking place on our side due to the contribution given to the establishment of VETistanbul Group. Known as, VETistanbul Group was established, under the coordination of Istanbul University, with joint decision of Veterinary Faculty of the University of Sarajevo, Saint Petersburg State Academy of Veterinary Medicine, Stara Zagora Trakia University, Ss. -

C O N F E R E N C E 22 27 April 2016
Joint Pathology Center Veterinary Pathology Services WEDNESDAY SLIDE CONFERENCE 2015-2016 C o n f e r e n c e 22 27 April 2016 Cory Brayton, DVM, Ph.D., DACVP Associate Professor, Molecular & Comparative Pathobiology Johns Hopkins University School of Medicine Broadway Research Building, Suite 851 733 North Broadway Baltimore, MD 21205 CASE I: NIEHS-087 (JPC 4017222). Signalment: 11-month-old B6.129S- Cybbtm1Din/J mouse (Mus musculus) History: A breeding colony of B6.129S- Cybbtm1Din/J mice were housed in an AAALAC International accredited facility. The mice were housed in static micro isolator cases with ad libitum autoclaved food (NIH-31) and beta chip bedding. Mice were provided acidified water due to imm- unocompromised state. The mice were Body as a while, mouse. The liver was slightly enlarged, housed in the same room as B6 imm- and there are multiple tan foci in the liver and lung. (Photo courtesy of: National Institute of Environmental unocompetent mice. Sudden deaths were Health Sciences, Cellular and Molecular Pathology noted in the colony over a weekend. A total Branch and Comparative Medicine Branch, P.O. Box of 87 mice, aged from one to eleven months 12233, Research Triangle Park, NC 27709, http://www.niehs.nih.gov/research/atniehs/labs/lep/index. were affected. Of these, 45 mice were found cfm) dead and 19 sick mice were euthanized and were multifocal tan foci in the liver, spleen necropsied. Twenty males and 38 females and lung. were affected. Laboratory Results: From multiple tissues, Gross Pathology: The livers were pale and a pure culture of Burkholderia spp. -

Journal of Clinical Microbiology
JOURNAL OF CLINICAL MICROBIOLOGY Volume 45 September 2007 No. 9 MINIREVIEW 16S rRNA Gene Sequencing for Bacterial Identification in J. Michael Janda and Sharon L. 2761–2764 the Diagnostic Laboratory: Pluses, Perils, and Pitfalls Abbott BACTERIOLOGY Is the Volume of Blood Cultured Still a Significant Factor in Emilio Bouza, Dolores Sousa, 2765–2769 the Diagnosis of Bloodstream Infections? Marta Rodrı´guez-Cre´ixems, Juan Garcı´a Lechuz, and Patricia Mun˜oz Reclassification of Phenotypically Identified Staphylococcus Takashi Sasaki, Ken Kikuchi, 2770–2778 intermedius Strains Yoshikazu Tanaka, Namiko Takahashi, Shinichi Kamata, and Keiichi Hiramatsu Evaluation of Gen-Probe APTIMA-Based Neisseria Erik Munson, Vivian Boyd, Jolanta 2793–2797 gonorrhoeae and Chlamydia trachomatis Confirmatory Testing Czarnecka, Judy Griep, Brian Lund, in a Metropolitan Setting of High Disease Prevalence Nancy Schaal, and Jeanne E. Hryciuk Convenient Test Using a Combination of Chelating Agents Soo-Young Kim, Seong Geun Hong, 2798–2801 for Detection of Metallo--Lactamases in the Clinical Ellen S. Moland, and Kenneth S. Laboratory Thomson Molecular Characterization of Vancomycin-Resistant Bo Zheng, Haruyoshi Tomita, Yong 2813–2818 Enterococcus faecium Isolates from Mainland China Hong Xiao, Shan Wang, Yun Li, and Yasuyoshi Ike Bacteriology of Moderate-to-Severe Diabetic Foot Infections Diane M. Citron, Ellie J. C. 2819–2828 and In Vitro Activity of Antimicrobial Agents Goldstein, C. Vreni Merriam, Benjamin A. Lipsky, and Murray A. Abramson Outbreak of Pseudomonas -

Westchester County Restaurants
RESTAURANTS THAT ARE ENOUGH TO MAKE YOU SICK: AN ANALYSIS OF UNSANITARY CONDITIONS AT NEW YORK CITY AND WESTCHESTER COUNTY RESTAURANTS STATE SENATOR JEFF KLEIN RANKING MINORITY MEMBER CONSUMER PROTECTION COMMITTEE RESTAURANTS ENOUGH TO MAKE YOU SICK: AN ANALYSIS OF UNSANITARY CONDITIONS AT NEW YORK CITY AND WESTCHESTER COUNTY RESTAURANT S How Consumers Are At Risk There is little reason why eating a meal in a restaurant should be any more dangerous than eating a meal in your own home. The volume and variety of foods prepared in a restaurant kitchen makes sanitation more critical, but the basic rules of cleanliness, temperature control and pest control are universal. The major difference is that the restaurant patron cannot usually see the kitchen where his or her meal is prepared. These unseen risks are present and they can pose a serious threat to the public. Worse, they may go undetected and unaddressed for extended periods of time. Although inspections are the first step toward catching these problems before they become public health hazards, many problems linger long after they have been cited by inspectors because restaurants can continue to operate with ongoing violations, with no warning to consumers. Many of the violations we have looked at, and many of the most common violations, are easily correctable, and if eradicated, greatly reduce the probability of an outbreak of food borne illness. By simply ensuring that potentially hazardous foods are properly stored, that they do not come into contact with other ready-to-eat foods, and ensuring that employees wash their hand or change their gloves after handling such foods would almost eliminate the risks posed by these foods. -

Supplemental Material S1.Pdf
Phylogeny of Selenophosphate synthetases (SPS) Supplementary Material S1 ! SelD in prokaryotes! ! ! SelD gene finding in sequenced prokaryotes! We downloaded a total of 8263 prokaryotic genomes from NCBI (see Supplementary Material S7). We scanned them with the program selenoprofiles (Mariotti 2010, http:// big.crg.cat/services/selenoprofiles) using two SPS-family profiles, one prokaryotic (seld) and one mixed eukaryotic-prokaryotic (SPS). Selenoprofiles removes overlapping predictions from different profiles, keeping only the prediction from the profile that seems closer to the candidate sequence. As expected, the great majority of output predictions in prokaryotic genomes were from the seld profile. We will refer to the prokaryotic SPS/SelD !genes as SelD, following the most common nomenclature in literature.! To be able to inspect results by hand, and also to focus on good-quality genomes, we considered a reduced set of species. We took the prok_reference_genomes.txt list from ftp://ftp.ncbi.nlm.nih.gov/genomes/GENOME_REPORTS/, which NCBI claims to be a "small curated subset of really good and scientifically important prokaryotic genomes". We named this the prokaryotic reference set (223 species - see Supplementary Material S8). We manually curated most of the analysis in this set, while we kept automatized the !analysis on the full set.! We detected SelD proteins in 58 genomes (26.0%) in the prokaryotic reference set (figure 1 in main paper), which become 2805 (33.9%) when considering the prokaryotic full set (figure SM1.1). The difference in proportion between the two sets is due largely to the presence of genomes of very close strains in the full set, which we consider redundant. -

Co-Infection Associated with Diarrhea in a Colony of <I>Scid
Laboratory Animal Science Vol 48, No 5 Copyright 1998 October 1998 by the American Association for Laboratory Animal Science Helicobacter bilis/Helicobacter rodentium Co-Infection Associated with Diarrhea in a Colony of scid Mice Nirah H. Shomer,* Charles A. Dangler, Robert P. Marini, and James G. Fox† Abstract _ An outbreak of diarrhea spanning 3 months occurred in a breeding colony of scid/Trp53 knockout mice. Approximately a third of the 150 mice were clinically affected, with signs ranging from mucoid or watery diarrhea to severe hemorrhagic diarrhea with mortality. Helicobacter bilis and the newly recognized urease-negative organ- ism H. rodentium were isolated from microaerobic culture of feces or cecal specimens from affected mice. Dual infection with H. bilis and H. rodentium were confirmed by culture and polymerase chain reaction (PCR) in several animals. Both Helicobacter species rapidly colonized immunocompetent sentinel mice exposed to bedding from cages containing affected mice, but the sentinel remained asymptomatic. Mice with diarrhea had multifocal to segmental proliferative typhlitis, colitis, and proctitis. Several affected mice had multifocal mucosal necrosis with a few focal ulcers in the cecum, colon, and rectum. Mice with diarrhea were treated with antibiotic food wafers (1.5 mg of amoxicillin, 0.69 mg of metronidazole, and 0.185 mg of bismuth/mouse per day) previously shown to eradi- cate H. hepaticus in immunocompetent mice. Antibiotic treatment resulted in resolution of diarrhea, but not eradication of H. bilis and H. rodentium; mice continued to have positive PCR results after a 2-week treatment regimen, and clinical signs of diarrhea returned in some mice when treatment was suspended. -

Enterohepatic Lesions in SCID Mice Infected with Helicobacter Bilis
Laboratory Animal Science Vol 48, No 4 Copyright 1998 August 1998 by the American Association for Laboratory Animal Science Enterohepatic Lesions in SCID Mice Infected with Helicobacter bilis Craig L. Franklin, Lela K. Riley, Robert S. Livingston, Catherine S. Beckwith, Cynthia L. Besch-Williford, and Reuel R. Hook, Jr. Abstract _ Helicobacter bilis is a recently identified species that colonizes the intestine and liver of mice. In immunocompetent mice, infections have been associated with mild hepatitis, and in immunocompromised mice, inflammatory bowel disease has been induced by intraperitoneal inoculation of the organism. We re- port inoculation of 6-week-old C.B-17 scid/scid mice by gastric gavage with approximately 107 H. bilis colony- forming units. Groups of mice were euthanized and necropsied 12, 24, and 36 weeks after inoculation. Mild to moderate proliferative typhlitis was evident in all mice at 12 and 36 weeks after inoculation and in most mice 24 weeks after inoculation. Mild to severe chronic active hepatitis was detected in 10 of 10 male mice and 3 of 10 female mice. These results indicate that H. bilis can cause moderate to severe enterohepatic disease in immunocompromised mice. The genus Helicobacter is a rapidly expanding genus volved in lesion development. Culture of specimens from currently containing 17 named species. Members of this mice confirmed intestinal colonization with H. hepaticus. Fox genus are microaerophilic, have curved to spiral rod mor- et al. reported enteric lesions in immunocompetent germ- phology, and are motile by flagella that vary in number free Swiss Webster mice infected with H. hepaticus (15), and and location among various species (1). -

Biliary Tract Microbiota: a New Kid on the Block of Liver Diseases?
European Review for Medical and Pharmacological Sciences 2020; 24: 2750-2775 Biliary tract microbiota: a new kid on the block of liver diseases? A. NICOLETTI1, F.R. PONZIANI2, E. NARDELLA1, G. IANIRO2, A. GASBARRINI1, L. ZILERI DAL VERME2 1Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy 2Internal Medicine, Gastroenterology and Hepatology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy Abstract. – The microbiome plays a crucial man body1,2. Indeed, a resident microbiota has recent- role in maintaining the homeostasis of the or- ly been described in several human environments ganism. Recent evidence has provided novel previously described as devoid of microorganisms, insights for understanding the interaction be- such as the urinary tract and the stomach3-9. Even tween the microbiota and the host. However, the 10 vast majority of such studies have analyzed the healthy placenta hosts microbial communities . interactions taking place in the intestinal tract. Bile has traditionally been considered sterile The biliary tree has traditionally been consid- under normal conditions11-14. ered sterile under normal conditions. However, The physical and chemical features of bile and the advent of metagenomic techniques has re- its antimicrobial activity were supposed to create vealed an unexpectedly rich bacterial communi- a hostile environment for bacteria. Moreover, the ty in the biliary tract. Associations between specific microbiolog- difficulty in collecting bile samples, coupled with ical patterns and inflammatory biliary diseases the lack of sensibility of culture techniques in and cancer have been recently described. Hence, detecting microbes in low-charge samples, sus- biliary dysbiosis may be a primary trigger in the tained this hypothesis for a long time. -

Helicobacter Spp. — Food- Or Waterborne Pathogens?
FRI FOOD SAFETY REVIEWS Helicobacter spp. — Food- or Waterborne Pathogens? M. Ellin Doyle Food Research Institute University of Wisconsin–Madison Madison WI 53706 Contents34B Introduction....................................................................................................................................1 Virulence Factors ...........................................................................................................................2 Associated Diseases .......................................................................................................................2 Gastrointestinal Disease .........................................................................................................2 Neurological Disease..............................................................................................................3 Other Diseases........................................................................................................................4 Epidemiology.................................................................................................................................4 Prevalence..............................................................................................................................4 Transmission ..........................................................................................................................4 Summary .......................................................................................................................................5 -

Helicobacter Hepaticus Model of Infection: the Human Hepatocellular Carcinoma Controversy
Review articles Helicobacter hepaticus model of infection: the human hepatocellular carcinoma controversy Yessica Agudelo Zapata, MD,1 Rodrigo Castaño Llano, MD,2 Mauricio Corredor, PhD.3 1 Medical Doctor and General Surgeon in the Abstract Gastrohepatology Group at Universidad de Antioquia in Medellin, Colombia The discovery of Helicobacter 30 years ago by Marshall and Warren completely changed thought about peptic 2 Gastrointestinal Surgeon and Endoscopist in the and duodenal ulcers. The previous paradigm posited the impossibility of the survival of microorganisms in the Gastrohepatology Group at the Universidad de stomach’s low pH environment and that, if any microorganisms survived, they would stay in the duodenum Antioquia in Medellin, Colombia 3 Institute Professor of Biology in the Faculty of or elsewhere in the intestine. Today the role of H. pylori in carcinogenesis is indisputable, but little is known Natural Sciences and the GEBIOMIC Group the about other emerging species of the genus Helicobacter in humans. Helicobacter hepaticus is one of these Gastroenterology Group at the Universidad de species that has been studied most, after H. pylori. We now know about their microbiological, genetic and Antioquia in Medellin, Colombia pathogenic relationships with HCC in murine and human infections. This review aims to show the medical and ......................................... scientifi c community the existence of new species of Helicobacter that have pathogenic potential in humans, Received: 07-05-13 thus encouraging research. Accepted: 27-08-13 Keywords Helicobacter hepaticus, Helicobacter pylori, Helicobacter spp., hepatocellular carcinoma. INTRODUCTION bacterium can infect animals including mice, dogs and ger- bils which could lead to a proposal to use H. -

Genomic Analysis of Helicobacter Himalayensis Sp. Nov. Isolated from Marmota Himalayana
Genomic analysis of Helicobacter himalayensis sp. nov. isolated from Marmota himalayana Shouhui Hu Peking University Shougang Hospital Lina Niu Hainan Medical University Lei Wu Peking University Shougang Hospital Xiaoxue Zhu Peking University Shougang Hospital Yu Cai Peking University Shougang Hospital Dong Jin Chinese Center for Disease Control and Prevention Linlin Yan Peking University Shougang Hospital Fan Zhao ( [email protected] ) Peking University Shougang Hospital https://orcid.org/0000-0002-8164-5016 Research article Keywords: Helicobacter, Comparative genomics, Helicobacter himalayensis, Virulence factor Posted Date: December 1st, 2020 DOI: https://doi.org/10.21203/rs.3.rs-55448/v3 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on November 23rd, 2020. See the published version at https://doi.org/10.1186/s12864-020-07245-y. Page 1/18 Abstract Background: Helicobacter himalayensis was isolated from Marmota himalayana in the Qinghai-Tibet Plateau, China, and is a new non-H. pylori species, with unclear taxonomy, phylogeny, and pathogenicity. Results: A comparative genomic analysis was performed between the H. himalayensis type strain 80(YS1)T and other the genomes of Helicobacter species present in the National Center for Biotechnology Information (NCBI) database to explore the molecular evolution and potential pathogenicity of H. himalayensis. H. himalayensis 80(YS1)T formed a clade with H. cinaedi and H. hepaticus that was phylogenetically distant from H. pylori. The H. himalayensis genome showed extensive collinearity with H. hepaticus and H. cinaedi. However, it also revealed a low degree of genome collinearity with H. -
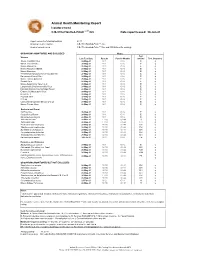
2021 ARC Health Report
Animal Health Monitoring Report Isolator reared C.B-17/IcrHanHsd-Prkdc scid /Arc Date report issued: 30-Jun-21 Report covers the following isolators: RI 27 Strains present in isolator: C.B-17/IcrHanHsd-Prkdc scid /Arc Strain of animal tested: C.B-17/IcrHanHsd-Prkdc scid /Arc and ICR Outbred for serology ORGANISMS MONITORED AND EXCLUDED Mouse Test Viruses Last Test Date Results Past 18 Months method Test frequency Mouse Hepatitis Virus 26-May-21 0 / 1 0 / 6 E q Minute Virus of Mice 26-May-21 0 / 1 0 / 6 E q Mouse Parvovirus 26-May-21 0 / 1 0 / 6 E q Murine Rotavirus (EDIM) 26-May-21 0 / 1 0 / 6 E q Mouse Norovirus 26-May-21 0 / 1 0 / 6 E q Theiler's Encephalomyelitis Virus (GD VII) 26-May-21 0 / 1 0 / 6 E q Pneumonia Virus of Mice 26-May-21 0 / 1 0 / 6 E q Murine Cytomegalovirus 26-May-21 0 / 1 0 / 6 E q Sendai Virus 26-May-21 0 / 1 0 / 6 E q Mouse Adenovirus Type 1 & 2 26-May-21 0 / 1 0 / 6 E q Lymphocytic Choriomeningitis Virus 26-May-21 0 / 1 0 / 6 E q Hantaan (Korean Haemorrhagic Fever) 26-May-21 0 / 1 0 / 6 E q Ectromelia (Mousepox) Virus 26-May-21 0 / 1 0 / 6 E q Reovirus -3 26-May-21 0 / 1 0 / 6 E q Polyoma Virus 26-May-21 0 / 1 0 / 6 E q K Virus 26-May-21 0 / 1 0 / 6 E q Lactic Dehydrogenase Elevating Virus 26-May-21 0 / 1 0 / 6 E q Mouse Thymic Virus 26-May-21 0 / 1 0 / 6 E q 00-Jan-00 Bacteria and Fungi CAR bacillus 26-May-21 0 / 1 0 / 6 E q Clostridium piliforme 26-May-21 0 / 1 0 / 6 E q Mycoplasma pulmonis 26-May-21 0 / 1 0 / 6 E q Helicobacter spp.1 26-May-21 0 / 10 0 / 44 H q Salmonella spp.